November 1, 2021

Four Success Stories in Gene Therapy
The field is beginning to fulfill its potential. These therapies offer a glimpse of what’s to come
By Jim Daley

Design Cells Getty Images
After numerous setbacks at the turn of the century, gene therapy is treating diseases ranging from neuromuscular disorders to cancer to blindness. The success is often qualified, however. Some of these therapies have proved effective at alleviating disease but come with a high price tag and other accessibility issues: Even when people know that a protocol exists for their disease and even if they can afford it or have an insurance company that will cover the cost—which can range from $400,000 to $2 million—they may not be able to travel to the few academic centers that offer it. Other therapies alleviate symptoms but don’t eliminate the underlying cause.
“Completely curing patients is obviously going to be a huge success, but it’s not [yet] an achievable aim in a lot of situations,” says Julie Crudele, a neurologist and gene therapy researcher at the University of Washington. Still, even limited advances pave the way for ongoing progress, she adds, pointing to research in her patients who have Duchenne muscular dystrophy: “In most of these clinical trials, we learn important things.”
Thanks to that new knowledge and steadfast investigations, gene therapy researchers can now point to a growing list of successful gene therapies. Here are four of the most promising.
Gene Swaps to Prevent Vision Loss
Some babies are born with severe vision loss caused by retinal diseases that once led inevitably to total blindness. Today some of them can benefit from a gene therapy created by wife-and-husband team Jean Bennett and Albert Maguire, who are now ophthalmologists at the University of Pennsylvania.
When the pair first began researching retinal disease in 1991, none of the genes now known to cause vision loss and blindness had been identified. In 1993 researchers identified one potential target gene, RPE65 . Seven years later Bennett and Maguire tested a therapy targeting that gene in three dogs with severe vision loss—it restored vision for all three.
In humans, the inherited condition that best corresponds with the dogs’ vision loss is Leber congenital amaurosis (LCA). LCA prevents the retina, a layer of light-sensitive cells at the back of the eye, from properly reacting or sending signals to the brain when a photon strikes it. The condition can cause uncontrolled shaking of the eye (nystagmus), prevents pupils from responding to light and typically results in total blindness by age 40. Researchers have linked the disease to mutations or deletions in any one of 27 genes associated with retinal development and function. Until gene therapy, there was no cure.
Mutations in RPE65 are just one cause of inherited retinal dystrophy, but it was a cause that Bennett and Maguire could act on. The researchers used a harmless adeno-associated virus (AAV), which they programmed to find retinal cells and insert a healthy version of the gene, and injected it into a patient’s eye directly underneath the retina. In 2017, after a series of clinical trials, the Food and Drug Administration approved voretigene neparvovecrzyl (marketed as Luxturna) for the treatment of any heritable retinal dystrophy caused by the mutated RPE65 gene, including LCA type 2 and retinitis pigmentosa, another congenital eye disease that affects photoreceptors in the retina. Luxturna was the first FDA-approved in vivo gene therapy, which is delivered to target cells inside the body (previously approved ex vivo therapies deliver the genetic material to target cells in samples collected from the body, which are then reinjected).
Spark Therapeutics, the company that makes Luxturna, estimates that about 6,000 people worldwide and between 1,000 and 2,000 in the U.S. may be eligible for its treatment—few enough that Luxturna was granted “orphan drug” status, a designation that the FDA uses to incentivize development of treatments for rare diseases. That wasn’t enough to bring the cost down. The therapy is priced at about $425,000 per injection, or nearly $1 million for both eyes. Despite the cost, Maguire says, “I have not yet seen anybody in the U.S. who hasn’t gotten access based on inability to pay.”
Those treated show significant improvement: Patients who were once unable to see clearly had their vision restored, often very quickly. Some reported that, after the injections, they could see stars for the first time.
While it is unclear how long the effects will last, follow-up data published in 2017 showed that all 20 patients treated with Luxturna in a phase 3 trial had retained their improved vision three years later. Bennett says five-year follow-up with 29 patients, which is currently undergoing peer review, showed similarly successful results. “These people can now do things they never could have dreamed of doing, and they’re more independent and enjoying life.”
Training the Immune System to Fight Cancer
Gene therapy has made inroads against cancer, too. An approach known as chimeric antigen receptor (CAR) T cell therapy works by programming a patient’s immune cells to recognize and target cells with cancerous mutations. Steven Rosenberg, chief of surgery at the National Cancer Institute, helped to develop the therapy and published the first successful results in a 2010 study for the treatment of lymphoma.
“That patient had massive amounts of disease in his chest and his belly, and he underwent a complete regression,” Rosenberg says—a regression that has now lasted 11 years and counting.
CAR T cell therapy takes advantage of white blood cells, called T cells, that serve as the first line of defense against pathogens. The approach uses a patient’s own T cells, which are removed and genetically altered so they can build receptors specific to cancer cells. Once infused back into the patient, the modified T cells, which now have the ability to recognize and attack cancerous cells, reproduce and remain on alert for future encounters.
In 2016 researchers at the University of Pennsylvania reported results from a CAR T cell treatment, called tisagenlecleucel, for acute lymphoblastic leukemia (ALL), one of the most common childhood cancers. In patients with ALL, mutations in the DNA of bone marrow cells cause them to produce massive quantities of lymphoblasts, or undeveloped white blood cells, which accumulate in the bloodstream. The disease progresses rapidly: adults face a low likelihood of cure, and fewer than half survive more than five years after diagnosis.
When directed against ALL, CAR T cells are ruthlessly efficient—a single modified T cell can kill as many as 100,000 lymphoblasts. In the University of Pennsylvania study, 29 out of 52 ALL patients treated with tisagenlecleucel went into sustained remission. Based on that study’s results, the FDA approved the therapy (produced by Novartis as Kymriah) for treating ALL, and the following year the agency approved it for use against diffuse large B cell lymphoma. The one-time procedure costs upward of $475,000.
CAR T cell therapy is not without risk. It can cause severe side effects, including cytokine release syndrome (CRS), a dangerous inflammatory response that ranges from mild flulike symptoms in less severe cases to multiorgan failure and even death. CRS isn’t specific to CAR T therapy: Researchers first observed it in the 1990s as a side effect of antibody therapies used in organ transplants. Today, with a combination of newer drugs and vigilance, doctors better understand how far they can push treatment without triggering CRS. Rosenberg says that “we know how to deal with side effects as soon as they occur, and serious illness and death from cytokine release syndrome have dropped drastically from the earliest days.”
Through 2020, the remission rate among ALL patients treated with Kymriah was about 85 percent. More than half had no relapses after a year. Novartis plans to track outcomes of all patients who received the therapy for 15 years to better understand how long it remains effective.
Precision Editing for Blood Disorders
One new arrival to the gene therapy scene is being watched particularly closely: in vivo gene editing using a system called CRISPR, which has become one of the most promising gene therapies since Jennifer Doudna and Emmanuelle Charpentier discovered it in 2012—a feat for which they shared the 2020 Nobel Prize in Chemistry. The first results from a small clinical trial aimed at treating sickle cell disease and a closely related disorder, called beta thalassemia, were published this past June.
Sickle cell disease affects millions of people worldwide and causes the production of crescent-shaped red blood cells that are stickier and more rigid than healthy cells, which can lead to anemia and life-threatening health crises. Beta thalassemia, which affects millions more, occurs when a different mutation causes someone’s body to produce less hemoglobin, the iron-rich protein that allows red blood cells to carry oxygen. Bone marrow transplants may offer a cure for those who can find matching donors, but otherwise treatments for both consist primarily of blood transfusions and medications to treat associated complications.
Both sickle cell disease and beta thalassemia are caused by heritable, single-gene mutations, making them good candidates for gene-editing therapy. The method, CRISPR-Cas9, uses DNA sequences from bacteria (clustered regularly interspaced short palindromic repeats, or CRISPR) and a CRISPR-associated enzyme (Cas for short) to edit the patient’s genome. The CRISPR sequences are transcribed onto RNA that locates and identifies DNA sequences to blame for a particular condition. When packaged together with Cas9, transcribed RNA locates the target sequence, and Cas9 snips it out of the DNA, thereby repairing or deactivating the problematic gene.
At a conference this past June, Vertex Pharmaceuticals and CRISPR Therapeutics announced unpublished results from a clinical trial of beta thalassemia and sickle cell patients treated with CTX001, a CRISPR-Cas9-based therapy. In both cases, the therapy does not shut off a target gene but instead delivers a gene that boosts production of healthy fetal hemoglobin—a gene normally turned off shortly after birth. Fifteen people with beta thalassemia were treated with CTX001; after three months or more, all 15 showed rapidly improved hemoglobin levels and no longer required blood transfusions. Seven people with severe sickle cell disease received the same treatment, all of whom showed increased levels of hemoglobin and reported at least three months without severe pain. More than a year later those improvements persisted in five subjects with beta thalassemia and two with sickle cell. The trial is ongoing, and patients are still being enrolled. A Vertex spokesperson says it hopes to enroll 45 patients in all and file for U.S. approval as early as 2022.
Derailing a Potentially Lethal Illness
Spinal muscular atrophy (SMA) is a neurodegenerative disease in which motor neurons—the nerves that control muscle movement and that connect the spinal cord to muscles and organs—degrade, malfunction and die. It is typically diagnosed in infants and toddlers. The underlying cause is a genetic mutation that inhibits production of a protein involved in building and maintaining those motor neurons.
The four types of SMA are ranked by severity and related to how much motor neuron protein a person’s cells can still produce. In the most severe or type I cases, even the most basic functions, such as breathing, sitting and swallowing, prove extremely challenging. Infants diagnosed with type I SMA have historically had a 90 percent mortality rate by one year.
Adrian Krainer, a biochemist at Cold Spring Harbor Laboratory, first grew interested in SMA when he attended a National Institutes of Health workshop in 1999. At the time, Krainer was investigating how RNA mutations cause cancer and genetic diseases when they disrupt a process called splicing, and researchers suspected that a defect in the process might be at the root of SMA. When RNA is transcribed from the DNA template, it needs to be edited or “spliced” into messenger RNA (mRNA) before it can guide protein production. During that editing process, some sequences are cut out (introns), and those that remain (exons) are strung together.
Krainer realized that there were similarities between the defects associated with SMA and one of the mechanisms he had been studying—namely, a mistake that occurs when an important exon is inadvertently lost during RNA splicing. People with SMA were missing one of these crucial gene sequences, called SMN1 .
“If we could figure out why this exon was being skipped and if we could find a solution for that, then presumably this could help all the [SMA] patients,” Krainer says. The solution he and his colleagues hit on, antisense therapy, employs single strands of synthetic nucleotides to deliver genetic instructions directly to cells in the body [see “ The Gene Fix ”]. In SMA’s case, the instructions induce a different motor neuron gene, SMN2 , which normally produces small amounts of the missing motor neuron protein, to produce much more of it and effectively fill in for SMN1 . The first clinical trial to test the approach began in 2010, and by 2016 the FDA approved nusinersen (marketed as Spinraza). Because the therapy does not incorporate itself into the genome, it must be administered every four months to maintain protein production. And it is staggeringly expensive: a single Spinraza treatment costs as much as $750,000 in the first year and $375,000 annually thereafter.
Since 2016, more than 10,000 people have been treated with it worldwide. Although Spinraza can’t restore completely normal motor function (a single motor neuron gene just can’t produce enough protein for that), it can help children with any of the four types of SMA live longer and more active lives. In many cases, Spinraza has improved patients’ motor function, allowing even those with more severe cases to breathe, swallow and sit upright on their own. “The most striking results are in patients who are being treated very shortly after birth, when they have a genetic diagnosis through newborn screening,” Krainer says. “Then, you can actually prevent the onset of the disease—for several years and hopefully forever.”
This article is part of “ Innovations In: Gene Therapy ,” an editorially independent special report that was produced with financial support from Pfizer .

Interactive Resources for Schools
- Page 1 - Genetic engineering
- Page 2 - What is genetic engineering?
- Page 3 - How does genetic engineering work?
- Page 4 - Ways of moving genes
- Page 5 - CRISPR-Cas9: a game-changer
- Page 6 - Genetic engineering: hopes and fears I
- Page 7 - Genetic engineering: hopes and fears II
- Page 8 - Transgenic plants – food for the future
- Page 9 - Gene therapy – case studies
- Page 10 - Gene silencing
- Page 11 - Recombinant hormones
- Page 12 - Minimising the risks of genetic engineering
- Page 13 - Ethics, laws and religion
- Page 14 - Activity: genetic engineering in the media
- Page 15 - Activity: genetic engineering in commercial agriculture
Help and information
This topic takes on average 55 minutes to read.
There are a number of interactive features in this resource:
Glossary: Any word with a glossary entry is highlighted like this. Moving the mouse over the highlighted word will show a definition of that word.
Animations: This topic has features with which you can interact, these are usually animations. Most of the animations can be expanded to full screen size, on a new window, ideal for showing on an interactive whiteboard. The animations will play all the way through or can be viewed one section at a time.
Downloads: Teachers can download individual diagrams, animations and other content from the Download Library area of the website. Terms and Conditions apply.
Activity: We propose an activity to help the students further consolidate their understanding of the topic.
Topic last updated: 18 Nov 2021
You may also be interested in these related topics, biotechnology, genes and inheritance, genetic engineering, gene therapy – case studies, this page is recommended for 16+ students. 14-16 might consider this page as a stretch & challenge , as it is quite advanced..
SCID – also known as severe combined immunodeficiency – is a very rare genetic disorder which only affects between 1 in 50,000 and 1 in 100,000 births. Children born with SCID do not have an effective immune system , so they are extremely vulnerable to any form of infection. In many instances, all of the problems result from a single defective gene coding for the enzyme adenosine deaminase. Boys are more often affected than girls because at least one form of the disease is sex-linked (carried on the X chromosome ).
In the past, the only way of keeping these children alive was to bring them up in a completely sterile environment, with all their food, water and air sterilised and with no direct contact with other people. Even then, affected children rarely lived into their teens as the slightest contamination could kill them.
Another alternative is a bone marrow transplant if a suitable donor can be found. Although the affected child has no immune system to cause rejection , the transplanted marrow can attack the patient’s cells. What is more, the donor cells may be infected with a virus – and this can kill the recipient very quickly. Patients can also be regularly injected with the enzyme they need, but this involves a lifetime of carefully managed therapy.

Life for children with SCID without treatment is very limited.
So gene therapy , inserting a healthy gene into the DNA using a vector such as a specially modified virus, offers the exciting possibility of a normal life for children who otherwise have a limited life expectancy and relatively poor quality of life.
The first ever attempts at gene therapy were carried out on children with SCID. Different variations of the technique were tried on children in several countries, including Britain. The trials had considerable success – the children treated all developed functioning immune system s which enabled them to fight off infections and to make antibodies when they were given vaccines. They could leave hospital, and their sterile environments, and live normal lives.
Then came the news that, about 3 years after their treatment, first one and then two of the nine children with SCID treated successfully using gene therapy in France developed leukaemia -like symptoms. They responded well to chemotherapy , but both the French and the American governments halted trials of gene therapy for SCID until more was known about why these boys fell ill and whether it was linked to the gene therapy.
The UK government decided differently, feeling that the potential benefits outweighed the possible risks. This view was backed up both by doctors carrying out the therapy at Great Ormond Street Hospital and by the mother of Rhys Evans, the first British boy to be given gene therapy. He received the treatment in 2001, when he was an infant, and he is now a healthy young man, enjoying normal life with a functioning immune system. Great Ormond Street has had many success stories treating this extremely rare condition with gene therapy. They are now considering ways to use the same techniques to tackle other genetic diseases.
Professor Nevin, who chaired the UK committee which made the decision that work should continue commented: "As with all innovative treatments, there will always be the potential for side-effects."
Dr Bobby Gaspar of Great Ormond Street Hospital said: "If we stop these studies now we will be denying extremely effective therapy to children and they may suffer as a result of not receiving this therapy. Ethically we believe it is the right thing to go on."
Marie Evans, the mother of Rhys who has undergone the treatment, also had an opinion.
"If they stop something just because one child has an adverse effect at the end of the day medicine and the world just doesn't go on," she said.
Gene therapy isn’t suitable for all patients, but at Great Ormond Street a number of children have now been successfully treated, without developing leukaemia, and trials into other uses of the technology are underway.
Sickle cell disease
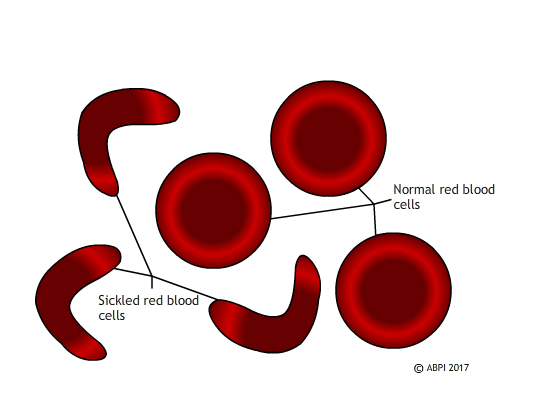
Sickled red blood cells do not carry oxygen effectively and they block small blood vessels. Gene therapy holds out the hope of dealing with both problems in one solution.
Unlike SCID, which is extremely rare, sickle cell disease affects millions of people around the world. In sickle cell disease, a mutation in a single gene affects the formation of one of the two types of protein chain which make up haemoglobin . This changes the shape of the haemoglobin molecule and reduces its ability to carry oxygen. The mutated haemoglobin also makes the red blood cells take on a sickle shape instead of the normal biconcave discs. These sickled red blood cells tend to stick together. They block small blood vessels , causing terrible pain and often tissue damage as well. People who are affected need regular blood transfusions, and often strong painkillers. Bone marrow transplant s can treat the disease, but only about 10% of the millions of people affected globally ever find a matching donor. Ultimately - and especially if untreated – sickle cell disease can kill.
In 2017 French scientists announced that they had reversed the progress of sickle cell disease in a teenage boy, by genetic modification of his bone marrow. The boy was very severely affected. By 13 he had had his spleen removed and his hips replaced, and he needed opioid painkillers to deal with the pain. Scientists took bone marrow stem cell s, genetically modified them using a viral vector so they could make functioning haemoglobin, and replaced the stem cells in the patient. For 15 months the boy has been making normal haemoglobin, and his red blood cells have functioned perfectly normally. He does not need transfusions or painkillers.
Scientists are always wary of claiming to have found a cure – and this patient is the first to succeed in the clinical trials. It will require many more years of testing – and success in other patients – before the procedure can be declared a complete success but this appears to be a major step forward. Seven other patients have been treated by the French team and they are also showing promising progress.
This is a very exciting development which could potentially help huge numbers of people – for example, 100,000 people are affected by sickle cell disease in the US alone. However, it also raises some ethical questions. The majority of people affected by sickle cell disease live in relatively poor countries, with limited health infrastructure. They do not have the resources to offer gene therapy to everyone – or even a minority – of the people affected. So at the moment, even if gene therapy does provide a cure for sickle cell disease, it will be a cure which is only available to affected people in the richer countries of the world. Perhaps as gene editing becomes more common and more successful it will become easier and cheaper and therefore available globally.
Perhaps we will need to find other ways of treating this and other genetic diseases. Whatever the future holds, we need to consider both the science and the ethics of the treatments we develop.
You can find out more about SCID and the use of gene therapy here: Treating the bubble babies: gene therapy in use, Your Genome Severe combined immunodeficiency , Great Ormond Street Hospital for Children Gene therapy success, Great Ormond Street Hospital for Children
Find out more about gene therapy and sickle cell disease here: Gene therapy ‘cures’ boy of blood disease that affects millions, New Scientist Teenager’s sickle cell reversed with world-first therapy, BBC News website
Muscular dystrophy – the importance of animal models
Duchenne muscular dystrophy (DMD) is the most severe form of muscular dystrophy. It affects about one in every 3500 boys who are born – about 100 boys a year in the UK. It is a sex-linked genetic condition which means the boys cannot make a protein called dystrophin, a protein vitally important for maintaining healthy muscles. Without it the muscles weaken and waste away, being replaced by fat, so that by their early teens most affected boys are confined to a wheelchair and their life expectancy is only to early adulthood.
The faulty gene is very large, which makes normal gene therapy techniques difficult. However researchers in the United States and in Britain have found ways of using parts of a healthy gene, called mini-genes, to repair the damaged DNA, enabling the muscles to produce dystrophin and to function in a much more normal way. What is more, the effect has been long term – the protein was still being made a year after the gene was inserted. The only problem is that the gene therapy technique has so far only been tried in mice and golden retrievers, which have a natural mutation similar to muscular dystrophy .
Much of this research depends on knockout mice. To produce knockout mice researchers genetically modify some embryo nic stem cell s to inactivate or ‘knock out’ a healthy gene. These cells are then injected into mouse embryos which are then implanted into a surrogate mother. The mice which result have some knockout cells and some normal cells, and they are then implanted to produce homozygous knockout mice.
Knockout mice often show changes in their phenotype which mimic human genetic problems , helping scientists understand exactly what the gene does.
Knockout mice are also useful for studying the impact of different therapies. We have many of our genes in common – of 4000 genes studied in mice and humans, only ten of them are found in one species but not in the other. This, along with the fact that mice reproduce rapidly, have large litters, and are easy and cheap to keep means that knockout mice are incredibly useful in our search to understand gene functions and to find cures for many diseases.
The problem with the mdx mice (a popular model for studying DMD) is that they only display relatively mild symptoms. Several breeds of domestic dog have also been found to have a natural mutation in the dystrophin gene and some work has been done on golden retrievers. Dogs are not ideal laboratory animals for many reasons – they are intelligent and emotive, they are not easy to manipulate genetically, and they take time and effort to breed. However, dogs affected by the canine form of Duchenne muscular dystrophy do have symptoms which are very similar to humans. Now a team at the Royal Veterinary College have discovered a line of King Charles spaniels which appear to have the same mutation in the same gene as humans. A research project began in 2015 looking at the progression of the disease in this breed of dog. This may in future lead to improved therapies for humans and dogs alike.

Many of the current trials on possible treatments for DMD still involve the use of medicines to alleviate symptoms, but there have been some promising results recently with genetic modification in both mice and dogs. A few phase 1 human clinical trials are in progress and more are expected soon. Some scientists are attempting to replace small regions of the faulty gene, others are trying to replace the whole thing. Gene therapy has not yet been fully successful in overcoming any genetic diseases, so any patients who take part in early trials of a possible new treatment – and their parents – are very brave. New technologies such as CRISPR-Cas9 hold out hope for new therapies including editing muscle-forming stem cell s rather than trying to change the whole organism. There is a long way to go, but muscular dystrophy is another disease where gene therapy may eventually result in a treatment or even a cure.
See: Knockout Mice Fact Sheet, National Human Genome Research Institute Why Mouse Matters, 2000 Mouse Sequencing Consortium, National Human Genome Research Institute A new animal model of Duchenne muscular dystrophy, Muscular Dystrophy UK
Discussion Point
Animals are frequently used in scientific research.
What are some arguments for and against this?
Transgenic plants – food for the future
Gene silencing.
- Gene Therapy
Gene Therapy Case Study: Cystic Fibrosis

Case Study: Gene Therapy for Enhancement Purposes
Dr. Anderson specializes in a particular type of gene therapy that targets Alzheimer’s Disease (AD).  Neural degeneration and synapse loss in the brain are characteristic of AD. Therefore, this gene therapy aims to protect neurons from degeneration and enhance the function of any neurons that are remaining. Dr. Anderson has two patients request her services. However, after an initial meeting with them, she is unsure whether she should treat them both.
Alexis is a 50 year-old woman who has a family history of AD and is already beginning to experience very mild symptoms of what she thinks is AD. She tells Dr. Anderson that her mother was afflicted with AD. So, she knows first-hand the sadness and frustration the family of an AD patient has to experience. Alexis has a husband and three children and does not want to put them through the same difficult journey. Therefore, she is requesting the gene therapy to reverse the small-scale symptoms she already has and prevent the onset of the disease.
Kelly is a 21 year-old college student who is applying for medical school in the very near future. Her academic history is strong but not exceptional. For this reason, Kelly fears that she will not be accepted to the top medical schools. Kelly wants to attend medical school so she can help underserved populations and work in impoverished areas that lack good healthcare. She tells Dr. Anderson that she would like to receive the Alzheimer’s gene therapy in hopes it will boost her memory and enhance neural function. Kelly believes a good score on the MCAT will strengthen her application and enable her to fulfill her dream of providing medical aid to the world’s neediest people.
Dr. Anderson decides to treat Alexis, as she feels that Alexis is the type of patient that the therapy is designed for. However, she conflicted about offering the treatment for Kelly. She doesn’t like the idea of withholding medical treatment from a patient, but the treatment was not originally intended for enhancement purposes.
Should Dr. Anderson treat Kelly?
- Yes. It is not the role of a doctor to make value judgments on who should and should not receive treatment. Ultimately, treating Kelly will benefit mankind when she becomes a doctor
- No. The treatment was designed to help patients that have AD to regain their normal function. Regardless of the reason, gene therapy should not be used for enhancement purposes.
View Results
Switch language:

Inside the efforts to rescue a rare disease gene therapy
Several patients are navigating ways to access innovative gene therapies with the help of enterprising research and nonprofit organisations.
- Share on Linkedin
- Share on Facebook

A parent’s worst nightmare is being told their child has been diagnosed with a rare disease. But being unable to access a possible cure makes it worse.
At seven months of age, Hailey and Jeff Barlow’s oldest daughter Jaylee was diagnosed a rare genetic disorder that causes severe immune deficiency and vulnerability to infections called adenosine deaminase severe combined immunodeficiency (ADA-SCiD). For treatment, Jaylee underwent a traditional bone marrow transplant after receiving a very high dose of chemotherapy. “It was such a traumatic experience for us,” shared Hailey.
Go deeper with GlobalData

NPVM: Alaunos Therapeutics Inc's Gene Therapy 2 for Oncology
Immuno-oncology in pharmaceuticals: gene therapy delivery using vir..., data insights.
The gold standard of business intelligence.
Find out more
Related Company Profiles
Orchard therapeutics plc.
However, Hailey and Jeff had heard about a gene therapy for ADA-SCiD – being co-developed by scientists at University College London (UCL) in the UK and University of California, Los Angeles (UCLA) in the US. Named simoladagene autotemcel, the therapy is not FDA approved and is only available via clinical trials. Previously named OTL-101, Orchard Therapeutics licensed the treatment from UCLA in 2016 to make it commercially available. However, in 2018, Orchard dropped the therapy after a pipeline reorganisation for financial reasons.
Dr. Donald Kohn, lead investigator of the ADA-SCiD programme at UCLA explained that families and doctors could no longer access the gene therapy after Orchard pulled out, and after increasing pressure from families, Orchard transferred the license back to UCLA in 2022. Clinical trials finally resumed in 2023 after the FDA requested amendments to the newly developed UCLA clinical protocol and manufacturing plans.
“We started keeping a list, although I didn’t know if it would ever mean anything. Once it [the gene therapy] came back to us, all of a sudden that list became the holy grail for families. Where was my child on the list? Will they be able to be treated?” said Kohn.
During her pregnancy, after Hailey learned that their child would be born with the condition through amniocentesis, she had gotten in contact with Kohn, and Hazel was placed on the waiting list for treatment before she was born. The family waited for four years for the clinical trial to restart, eventually going to UCLA to start the treatment process in January 2023.
How well do you really know your competitors?
Access the most comprehensive Company Profiles on the market, powered by GlobalData. Save hours of research. Gain competitive edge.

Your download email will arrive shortly
Not ready to buy yet? Download a free sample
We are confident about the unique quality of our Company Profiles. However, we want you to make the most beneficial decision for your business, so we offer a free sample that you can download by submitting the below form
Hailey said that they spent 10 days in hospital for the extraction and preparation of Hazel’s stem cells in the lab. In April 2023, they returned for another 10 days for Hazel to receive her stem cell transplant, following a small dose of chemotherapy.
“It has been almost a year and a half now since Hazel’s treatment and it was absolutely amazing how easy it was to go through the whole process. She never got sick. She never got down emotionally. And now she has a fully functioning immune system,” explained Hailey.
Establishing a framework to save gene therapies
Across the pond, the Great Ormond Street Hospital (GOSH) in the UK announced that it is now attempting to obtain the licence for UCLA-UCL’s therapy simoladagene autotemcel to treat ADA-SCiD on a non-profit basis, in April 2024. The importance of gaining full approval for these therapies was highlighted by Dr. Claire Booth, who leads the ADA-SCiD clinical trial at GOSH.
“Gene therapies, such as the one developed for ADA-SCiD between GOSH and UCLA, have been shown to be safe and very effective so we want to ensure that these proven treatments can be offered to patients and families quickly and simply, without the need to look at compassionate use approaches,” explained Booth.
It wouldn’t be the first time that an academic or non-profit organisation would have to ‘rescue’ a gene therapy . In September 2023, Italian research charity Telethon Foundation became a pioneer in this approach after it announced that it would manufacture and distribute a different gene therapy for ADA-SCiD, after that was also abandoned in a similar manner.
The gene therapy, dubbed Strimvelis, was developed by scientists of the San Raffaele-Telethon Institute for Gene Therapy in Milan, funded by the Telethon Foundation. In 2016, the European Medicines Agency (EMA) granted the therapy’s approval to GSK , who owned it at that time. GSK then sold the therapy to Orchard Therapeutics in 2018.
However, after Orchard reprioritised its plans in 2022, the biopharma transferred the therapy’s rights back to Telethon.
Francesca Pasinelli, general manager of Telethon Foundation explained that scientists at the San Raffaele-Telethon Institute for Gene Therapy were able to develop regulatory-grade data for the gene therapy programme, which allowed GSK to license it without having to repeat expensive early-stage studies.
“Opportunities for cross-leveraging existing data from other products remain limited and hence there is often duplication of activities, resulting in extended time and additional costs per product development programme which can significantly impact the commercial viability of certain diseases,” Pasinelli said. This type of partnership can help reduce the overall costs of gene therapy development, she explained.
Strimvelis works by editing the patients’ own hematopoietic stem cells with a functional version of the ADA gene which causes ADA-SCiD. The cells are then transferred back into the patient’s bone marrow to mature and produce the normal ADA protein.
Telethon worked closely with the manufacturing company AGC Biologics to develop and optimise the production process for their gene therapies. The enriched cells are still produced at the AGC pharmaceutical facility, located on the San Raffaele Campus of the Telethon Institute for Gene Therapy, and the treatment is administered exclusively at the San Raffaele Hospital.
Pasinelli emphasised that these types of partnerships should be complementary to the work done by pharmaceutical companies and that both non-profit and for-profit organisations have important roles to play in ensuring rare disease patients have access to effective gene therapies.
“This model can’t be unique, particularly for ultra rare diseases, where profits can be a challenge, even at very high prices. I think in the end, when you think of the very small number of patients, the real advantage is [in] saving lives,” said Pasinelli.
Nonetheless, regulatory requirements globally need to be harmonised to reduce redundant work and make the development process more efficient, while still maintaining high quality standards, adds Pasinelli.
In the coming months, the Telethon Foundation is going to apply for an EMA authorisation to market another gene therapy product originally developed in Milan—a treatment for Wiskott-Aldrich Syndrome, a rare congenital immunodeficiency.
The gene therapy, called OTL-103 (etuvetidigene autotemcel) also originated in the laboratories of the San Raffaele-Telethon Institute in Milan, and was the subject of an industrial partnership with Orchard. However, after the company divested from the program, in 2022, Telethon announced its commitment to obtain approval of this therapy by the regulatory authorities, making it available to patients.
“Rare diseases have been a very hot topic in the last few years. There are many conferences [where they are discussed], but at the end of the day, you have to provide patients with solutions.
“Patients cannot be the subject of the conference; they need to be someone we are really thinking of. We need a solution,” concluded Pasinelli.
Cell & Gene Therapy coverage on Pharmaceutical Technology is supported by Cytiva .
Editorial content is independently produced and follows the highest standards of journalistic integrity. Topic sponsors are not involved in the creation of editorial content.
Free Whitepaper
Cell and gene therapies: pipe dream to pipeline.
You will receive an email shortly. Please check your inbox to download the Whitepaper.

By downloading this case study, you acknowledge that GlobalData may share your information with Cytiva Thematic and that your personal data will be used as described in their Privacy Policy
Sign up for our daily news round-up!
Give your business an edge with our leading industry insights.
More Relevant
AbCellera and Lilly broadens antibody discovery partnership
Nice recommends beigene’s mzl tablet for nhs use, novo faces growth challenges despite glp-1 receptor agonist obesity boom, leveragen and moderna link to advance therapeutics, sign up to the newsletter: in brief, your corporate email address, i would also like to subscribe to:.
Pharma Technology Focus : Pharmaceutical Technology Focus (monthly)
Thematic Take (monthly)
I consent to Verdict Media Limited collecting my details provided via this form in accordance with Privacy Policy
Thank you for subscribing
View all newsletters from across the GlobalData Media network.
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Published: 29 July 2024
Genetically engineered hypoimmunogenic cell therapy
- Akitsu Hotta ORCID: orcid.org/0000-0002-2619-7441 1 ,
- Sonja Schrepfer 2 &
- Andras Nagy ORCID: orcid.org/0000-0003-4311-0413 3 , 4 , 5
Nature Reviews Bioengineering ( 2024 ) Cite this article
140 Accesses
8 Altmetric
Metrics details
- CRISPR-Cas9 genome editing
- Immune evasion
- Pluripotent stem cells
Immune rejection is an important obstacle to the use of allogeneic ‘off-the-shelf’ cells for transplantation into immunocompetent patients. Genetic modification has emerged as a promising approach to improve immune compatibility in various applications, including cancer immunotherapy and stem cell-based therapies. Several approaches have been proposed to evade the recognition and attack of transplanted cells by specific host immune cells; however, further investigation is needed to ensure the hypoimmunity and safety of cell-based therapies in clinical practice. This Review discusses key advances and challenges in the clinical translation of hypoimmunogenic cells and describes the genetic engineering methods and manufacturing processes used to create hypoimmunogenic therapeutic cells, while highlighting the complexity of relevant immunological pathways.
Hypoimmunogenic cells created through genetic engineering are likely to become a mainstream approach to allogeneic cell therapy that can avoid or mitigate the need for immunosuppression in treated patients.
Immune rejection of allogeneic cell-based clinical therapies can be avoided by the removal of antigens and/or the expression of immunosuppressive factors.
The potential risk of tumorigenesis can be mitigated by careful quality assessment and incorporation of a safety ‘kill switch’ into engineered cells.
To establish a successful hypoimmunogenic cell therapy, it is important to consider multiple factors, including which cell types to utilize, target genes, editing methods, quality control mechanisms and host-related factors.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
92,52 € per year
only 7,71 € per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others

Immunological barriers to haematopoietic stem cell gene therapy
Engineering universal cells that evade immune detection
Engineering immune-evasive allogeneic cellular immunotherapies
Elisseeff, J., Badylak, S. F. & Boeke, J. D. Immune and genome engineering as the future of transplantable tissue. N. Engl. J. Med. 385 , 2451–2462 (2021).
Article Google Scholar
Mihatsch, M. J. et al. The side-effects of ciclosporine-A and tacrolimus. Clin. Nephrol. 49 , 356–363 (1998).
Google Scholar
Miller, L. W. Cardiovascular toxicities of immunosuppressive agents. Am. J. Transplant. 2 , 807–818 (2002).
Taylor, A. L., Watson, C. J. & Bradley, J. A. Immunosuppressive agents in solid organ transplantation: mechanisms of action and therapeutic efficacy. Crit. Rev. Oncol. Hematol. 56 , 23–46 (2005).
Wood, K. J., Bushell, A. & Hester, J. Regulatory immune cells in transplantation. Nat. Rev. Immunol. 12 , 417–430 (2012).
Oberholtzer, N., Atkinson, C. & Nadig, S. N. Adoptive transfer of regulatory immune cells in organ transplantation. Front. Immunol. 12 , 631365 (2021).
Mikami, N. & Sakaguchi, S. Regulatory T cells in autoimmune kidney diseases and transplantation. Nat. Rev. Nephrol. 19 , 544–557 (2023).
Sakaguchi, S., Kawakami, R. & Mikami, N. Treg-based immunotherapy for antigen-specific immune suppression and stable tolerance induction: a perspective. Immunother. Adv. 3 , ltad007 (2023).
Kinsella, F. A. M. et al. Mixed chimerism established by hematopoietic stem cell transplantation is maintained by host and donor T regulatory cells. Blood Adv. 3 , 734–743 (2019).
Scandling, J. D. et al. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N. Engl. J. Med. 358 , 362–368 (2008).
Kawai, T. et al. HLA-mismatched renal transplantation without maintenance immunosuppression. N. Engl. J. Med. 358 , 353–361 (2008).
Bertaina, A. et al. Sequential stem cell–kidney transplantation in Schimke immuno-osseous dysplasia. N. Engl. J. Med. 386 , 2295–2302 (2022).
Ankrum, J. A., Ong, J. F. & Karp, J. M. Mesenchymal stem cells: immune evasive, not immune privileged. Nat. Biotechnol. 32 , 252–260 (2014).
Hutchinson, J. A. et al. Cutting edge: immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J. Immunol. 187 , 2072–2078 (2011).
Thomson, A. W., Metes, D. M., Ezzelarab, M. B. & Raïch-Regué, D. Regulatory dendritic cells for human organ transplantation. Transplant. Rev. 33 , 130–136 (2019).
Pang, S. H. M. et al. Mesenchymal stromal cell apoptosis is required for their therapeutic function. Nat. Commun. 12 , 6495 (2021).
Casiraghi, F., Perico, N. & Remuzzi, G. Mesenchymal stromal cells for tolerance induction in organ transplantation. Hum. Immunol. 79 , 304–313 (2018).
Tan, J. et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: a randomized controlled trial. J. Am. Med. Assoc. 307 , 1169–1177 (2012).
Detry, O. et al. Infusion of mesenchymal stromal cells after deceased liver transplantation: a phase I–II, open-label, clinical study. J. Hepatol. 67 , 47–55 (2017).
Wang, P. et al. Immune tolerance induction using cell-based strategies in liver transplantation: clinical perspectives. Front. Immunol. 11 , 1723 (2020).
Singh, A. et al. Long-term tolerance of islet allografts in nonhuman primates induced by apoptotic donor leukocytes. Nat. Commun. 10 , 3495 (2019).
Husain, I. & Luo, X. Apoptotic donor cells in transplantation. Front. Immunol. 12 , 626840 (2021).
Yu, S., Su, C. & Luo, X. Impact of infection on transplantation tolerance. Immunol. Rev. 292 , 243–263 (2019).
Wu, S. et al. Advances in encapsulation and delivery strategies for islet transplantation. Adv. Healthc. Mater. 10 , e2100965 (2021).
Barker, D. J. et al. The IPD-IMGT/HLA database. Nucleic Acids Res. 51 , D1053–D1060 (2023).
Wiebe, C. & Nickerson, P. Strategic use of epitope matching to improve outcomes. Transplantation 100 , 2048–2052 (2016).
Morishima, Y. et al. Biological significance of HLA locus matching in unrelated donor bone marrow transplantation. Blood 125 , 1189–1197 (2015).
Ayuk, F. et al. Relative impact of HLA matching and non-HLA donor characteristics on outcomes of allogeneic stem cell transplantation for acute myeloid leukemia and myelodysplastic syndrome. Biol. Blood Marrow Transplant. 24 , 2558–2567 (2018).
Süsal, C. & Opelz, G. Impact of HLA matching and HLA antibodies in organ transplantation: a collaborative transplant study view. Methods Mol. Biol. 882 , 267–277 (2012).
Opelz, G. & Döhler, B. Effect of human leukocyte antigen compatibility on kidney graft survival: comparative analysis of two decades. Transplantation 84 , 137–143 (2007).
Nikaein, A. et al. HLA compatibility and liver transplant outcome. Improved patient survival by HLA and cross-matching. Transplantation 58 , 786–792 (1994).
Opelz, G. & Wujciak, T. The influence of HLA compatibility on graft survival after heart transplantation. The collaborative transplant study. N. Engl. J. Med. 330 , 816–819 (1994).
Rudolph, E. N. et al. HLA-A, -B, -C, -DR, and -DQ matching in pancreas transplantation: effect on graft rejection and survival. Am. J. Transplant. 16 , 2401–2412 (2016).
Opelz, G., Süsal, C., Ruhenstroth, A. & Döhler, B. Impact of HLA compatibility on lung transplant survival and evidence for an HLA restriction phenomenon: a collaborative transplant study report. Transplantation 90 , 912–917 (2010).
Kanda, J. et al. Related transplantation with HLA-1 Ag mismatch in the GVH direction and HLA-8/8 allele-matched unrelated transplantation: a nationwide retrospective study. Blood 119 , 2409–2416 (2012).
Morishima, Y. et al. Impact of homozygous conserved extended HLA haplotype on single cord blood transplantation: lessons for induced pluripotent stem cell banking and transplantation in allogeneic settings. Biol. Blood Marrow Transplant. 26 , 132–138 (2020).
Yoshida, S. et al. A clinical-grade HLA haplobank of human induced pluripotent stem cells matching approximately 40% of the Japanese population. Med 4 , 51–66.e10 (2023).
Okita, K. et al. A more efficient method to generate integration-free human iPS cells. Nat. Methods 8 , 409–412 (2011).
Lee, S. et al. Repurposing the cord blood bank for haplobanking of HLA-homozygous iPSCs and their usefulness to multiple populations. Stem Cells 36 , 1552–1566 (2018).
Gourraud, P. A., Gilson, L., Girard, M. & Peschanski, M. The role of human leukocyte antigen matching in the development of multiethnic “haplobank” of induced pluripotent stem cell lines. Stem Cells 30 , 180–186 (2012).
Álvarez-Palomo, B. et al. Public cord blood banks as a source of starting material for clinical grade HLA-homozygous induced pluripotent stem cells. Stem Cell Res. Ther. 13 , 408 (2022).
Sugita, S. et al. HLA-matched allogeneic iPS cells-derived RPE transplantation for macular degeneration. J. Clin. Med. 9 , 2217 (2020).
Miyagawa, S. et al. Case report: transplantation of human induced pluripotent stem cell-derived cardiomyocyte patches for ischemic cardiomyopathy. Front. Cardiovasc. Med. 9 , 950829 (2022).
Mandai, M. Pluripotent stem cell-derived retinal organoid/cells for retinal regeneration therapies: a review. Regen. Ther. 22 , 59–67 (2023).
Morizane, A. Cell therapy for Parkinson’s disease with induced pluripotent stem cells. Inflamm. Regen. 43 , 16 (2023).
Sugai, K. et al. First-in-human clinical trial of transplantation of iPSC-derived NS/PCs in subacute complete spinal cord injury: study protocol. Regen. Ther. 18 , 321–333 (2021).
Deuse, T. et al. De novo mutations in mitochondrial DNA of iPSCs produce immunogenic neoepitopes in mice and humans. Nat. Biotechnol. 37 , 1137–1144 (2019).
Deuse, T. et al. SCNT-derived ESCs with mismatched mitochondria trigger an immune response in allogeneic hosts. Cell Stem Cell 16 , 33–38 (2015).
1000 Genomes Project Consortium et al. A global reference for human genetic variation. Nature 526 , 68–74 (2015).
Zhang, Q. & Reed, E. F. The importance of non-HLA antibodies in transplantation. Nat. Rev. Nephrol. 12 , 484–495 (2016).
Aron Badin, R. et al. MHC matching fails to prevent long-term rejection of iPSC-derived neurons in non-human primates. Nat. Commun. 10 , 4357 (2019).
Kawamura, T. et al. Cardiomyocytes derived from MHC-homozygous induced pluripotent stem cells exhibit reduced allogeneic immunogenicity in MHC-matched non-human primates. Stem Cell Rep. 6 , 312–320 (2016).
Lanza, R., Russell, D. W. & Nagy, A. Engineering universal cells that evade immune detection. Nat. Rev. Immunol. 19 , 723–733 (2019).
Petrus-Reurer, S. et al. Immunological considerations and challenges for regenerative cellular therapies. Commun. Biol. 4 , 798 (2021).
Doudna, J. A. The promise and challenge of therapeutic genome editing. Nature 578 , 229–236 (2020).
Anzalone, A. V., Koblan, L. W. & Liu, D. R. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38 , 824–844 (2020).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331 , 1565–1570 (2011).
O’Donnell, J. S., Teng, M. W. L. & Smyth, M. J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 16 , 151–167 (2019).
Münz, C. Latency and lytic replication in Epstein–Barr virus-associated oncogenesis. Nat. Rev. Microbiol. 17 , 691–700 (2019).
Ander, S. E., Diamond, M. S. & Coyne, C. B. Immune responses at the maternal-fetal interface. Sci. Immunol. 4 , eaat6114 (2019).
Torikai, H. et al. Toward eliminating HLA class I expression to generate universal cells from allogeneic donors. Blood 122 , 1341–1349 (2013).
Xu, H. et al. Targeted disruption of HLA genes via CRISPR-Cas9 generates iPSCs with enhanced immune compatibility. Cell Stem Cell 24 , 566–578.e7 (2019).
Parent, A. V. et al. Selective deletion of human leukocyte antigens protects stem cell-derived islets from immune rejection. Cell Rep. 36 , 109538 (2021).
Riolobos, L. et al. HLA engineering of human pluripotent stem cells. Mol. Ther. 21 , 1232–1241 (2013).
Rong, Z. et al. An effective approach to prevent immune rejection of human ESC-derived allografts. Cell Stem Cell 14 , 121–130 (2014).
Gornalusse, G. G. et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat. Biotechnol. 35 , 765–772 (2017).
Deuse, T. et al. Hypoimmunogenic derivatives of induced pluripotent stem cells evade immune rejection in fully immunocompetent allogeneic recipients. Nat. Biotechnol. 37 , 252–258 (2019).
Han, X. et al. Generation of hypoimmunogenic human pluripotent stem cells. Proc. Natl Acad. Sci. USA 116 , 10441–10446 (2019).
Deuse, T. et al. Hypoimmune induced pluripotent stem cell-derived cell therapeutics treat cardiovascular and pulmonary diseases in immunocompetent allogeneic mice. Proc. Natl Acad. Sci. USA 118 , e2022091118 (2021).
Harding, J. et al. Immune-privileged tissues formed from immunologically cloaked mouse embryonic stem cells survive long-term in allogeneic hosts. Nat. Biomed. Eng. 8 , 427–442 (2024).
Wani, M. A. et al. Familial hypercatabolic hypoproteinemia caused by deficiency of the neonatal Fc receptor, FcRn, due to a mutant β 2 -microglobulin gene. Proc. Natl Acad. Sci. USA 103 , 5084–5089 (2006).
Jo, S. et al. Endowing universal CAR T-cell with immune-evasive properties using TALEN-gene editing. Nat. Commun. 13 , 3453 (2022).
Haruta, M. et al. TAP-deficient human iPS cell-derived myeloid cell lines as unlimited cell source for dendritic cell-like antigen-presenting cells. Gene Ther. 20 , 504–513 (2013).
Hanna, S. & Etzioni, A. MHC class I and II deficiencies. J. Allergy Clin. Immunol. 134 , 269–275 (2014).
Reith, W. & Mach, B. The bare lymphocyte syndrome and the regulation of MHC expression. Annu. Rev. Immunol. 19 , 331–373 (2001).
Steimle, V., Otten, L. A., Zufferey, M. & Mach, B. Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome). Cell 75 , 135–146 (1993).
Kwon, Y. W. et al. HLA DR genome editing with TALENs in human iPSCs produced immune-tolerant dendritic cells. Stem Cells Int. 2021 , 8873383 (2021).
Madsen, L. et al. Mice lacking all conventional MHC class II genes. Proc. Natl Acad. Sci. USA 96 , 10338–10343 (1999).
Abrahimi, P. et al. Efficient gene disruption in cultured primary human endothelial cells by CRISPR/Cas9. Circ. Res. 117 , 121–128 (2015).
Mattapally, S. et al. Human leukocyte antigen class I and II knockout human induced pluripotent stem cell-derived cells: universal donor for cell therapy. J. Am. Heart Assoc. 7 , e010239 (2018).
Kwon, D. et al. Human leukocyte antigen class I pseudo-homozygous mesenchymal stem cells derived from human induced pluripotent stem cells. Stem Cell Rev. Rep. 16 , 792–808 (2020).
Kärre, K. Natural killer cell recognition of missing self. Nat. Immunol. 9 , 477–480 (2008).
Carretero, M. et al. Specific engagement of the CD94/NKG2-A killer inhibitory receptor by the HLA-E class Ib molecule induces SHP-1 phosphatase recruitment to tyrosine-phosphorylated NKG2-A: evidence for receptor function in heterologous transfectants. Eur. J. Immunol. 28 , 1280–1291 (1998).
Thielens, A., Vivier, E. & Romagné, F. NK cell MHC class I specific receptors (KIR): from biology to clinical intervention. Curr. Opin. Immunol. 24 , 239–245 (2012).
Rajagopalan, S. & Long, E. O. KIR2DL4 (CD158d): an activation receptor for HLA-G. Front. Immunol. 3 , 258 (2012).
Carr, W. H., Pando, M. J. & Parham, P. KIR3DL1 polymorphisms that affect NK cell inhibition by HLA-Bw4 ligand. J. Immunol. 175 , 5222–5229 (2005).
Hansasuta, P. et al. Recognition of HLA-A3 and HLA-A11 by KIR3DL2 is peptide-specific. Eur. J. Immunol. 34 , 1673–1679 (2004).
Winter, C. C., Gumperz, J. E., Parham, P., Long, E. O. & Wagtmann, N. Direct binding and functional transfer of NK cell inhibitory receptors reveal novel patterns of HLA-C allotype recognition. J. Immunol. 161 , 571–577 (1998).
Viant, C. et al. SHP-1-mediated inhibitory signals promote responsiveness and anti-tumour functions of natural killer cells. Nat. Commun. 5 , 5108 (2014).
Crew, M. D., Cannon, M. J., Phanavanh, B. & Garcia-Borges, C. N. An HLA-E single chain trimer inhibits human NK cell reactivity towards porcine cells. Mol. Immunol. 42 , 1205–1214 (2005).
Hu, X. et al. Hypoimmune induced pluripotent stem cells survive long term in fully immunocompetent, allogeneic rhesus macaques. Nat. Biotechnol. 42 , 413–423 (2024).
Braud, V. M. et al. HLA-E binds to natural killer cell receptors CD94/NKG2A, B and C. Nature 391 , 795–799 (1998).
Guethlein, L. A., Older Aguilar, A. M., Abi-Rached, L. & Parham, P. Evolution of killer cell Ig-like receptor ( KIR ) genes: definition of an orangutan KIR haplotype reveals expansion of lineage III KIR associated with the emergence of MHC-C. J. Immunol. 179 , 491–504 (2007).
Kitano, Y. et al. Generation of hypoimmunogenic induced pluripotent stem cells by CRISPR-Cas9 system and detailed evaluation for clinical application. Mol. Ther. Methods Clin. Dev. 26 , 15–25 (2022).
Ichise, H. et al. NK cell alloreactivity against KIR-ligand-mismatched HLA-haploidentical tissue derived from HLA haplotype-homozygous iPSCs. Stem Cell Rep. 9 , 853–867 (2017).
Kagita, A. et al. Efficient ssODN-mediated targeting by avoiding cellular inhibitory RNAs through precomplexed CRISPR-Cas9/sgRNA ribonucleoprotein. Stem Cell Rep. 16 , 985–996 (2021).
Parham, P. MHC class I molecules and KIRs in human history, health and survival. Nat. Rev. Immunol. 5 , 201–214 (2005).
Yawata, M. et al. MHC class I-specific inhibitory receptors and their ligands structure diverse human NK-cell repertoires toward a balance of missing self-response. Blood 112 , 2369–2380 (2008).
Wang, B. et al. Generation of hypoimmunogenic T cells from genetically engineered allogeneic human induced pluripotent stem cells. Nat. Biomed. Eng. 5 , 429–440 (2021).
Gardai, S. J. et al. Cell-surface calreticulin initiates clearance of viable or apoptotic cells through trans-activation of LRP on the phagocyte. Cell 123 , 321–334 (2005).
Deuse, T. et al. The SIRPα-CD47 immune checkpoint in NK cells. J. Exp. Med. 218 , e20200839 (2021).
Salaman, M. R. & Gould, K. G. Breakdown of T-cell ignorance: the tolerance failure responsible for mainstream autoimmune diseases? J. Transl. Autoimmun. 3 , 100070 (2020).
Kurts, C. et al. CD8 T cell ignorance or tolerance to islet antigens depends on antigen dose. Proc. Natl Acad. Sci. USA 96 , 12703–12707 (1999).
Calbo, S. et al. Functional tolerance of CD8 + T cells induced by muscle-specific antigen expression. J. Immunol. 181 , 408–417 (2008).
Hu, X. et al. Human hypoimmune primary pancreatic islets avoid rejection and autoimmunity and alleviate diabetes in allogeneic humanized mice. Sci. Transl. Med. 15 , eadg5794 (2023).
Hu, X. et al. Hypoimmune anti-CD19 chimeric antigen receptor T cells provide lasting tumor control in fully immunocompetent allogeneic humanized mice. Nat. Commun. 14 , 2020 (2023).
Hu, X. et al. Hypoimmune islets achieve insulin independence after allogeneic transplantation in a fully immunocompetent non-human primate. Cell Stem Cell 31 , 334–340.e5 (2024).
Coles, S. J. et al. CD200 expression suppresses natural killer cell function and directly inhibits patient anti-tumor response in acute myeloid leukemia. Leukemia 25 , 792–799 (2011).
Hoek, R. M. et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 290 , 1768–1771 (2000).
Ferreira, L. M. R., Meissner, T. B., Tilburgs, T. & Strominger, J. L. HLA-G: at the interface of maternal–fetal tolerance. Trends Immunol. 38 , 272–286 (2017).
Shi, L. et al. Generation of hypoimmunogenic human pluripotent stem cells via expression of membrane-bound and secreted β2m-HLA-G fusion proteins. Stem Cells 38 , 1423–1437 (2020).
Yi, Y. S. Functional role of milk fat globule-epidermal growth factor VIII in macrophage-mediated inflammatory responses and inflammatory/autoimmune diseases. Mediators Inflamm. 2016 , 5628486 (2016).
Comerford, I. et al. A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev. 24 , 269–283 (2013).
Francisco, L. M. et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 206 , 3015–3029 (2009).
Woodward, K. B. et al. Pancreatic islets engineered with a FasL protein induce systemic tolerance at the induction phase that evolves into long-term graft-localized immune privilege. Am. J. Transplant. 20 , 1285–1295 (2020).
Lenschow, D. J. et al. Long-term survival of xenogeneic pancreatic islet grafts induced by CTLA4lg. Science 257 , 789–792 (1992).
Ali, A. et al. CTLA4 immunoglobulin but not anti-tumor necrosis factor therapy promotes staphylococcal septic arthritis in mice. J. Infect. Dis. 212 , 1308–1316 (2015).
Riminton, D. S., Hartung, H. P. & Reddel, S. W. Managing the risks of immunosuppression. Curr. Opin. Neurol. 24 , 217–223 (2011).
Kahlig, K. M. et al. Multiplexed transposon-mediated stable gene transfer in human cells. Proc. Natl Acad. Sci. USA 107 , 1343–1348 (2010).
Woltjen, K. et al. piggyBac transposition reprograms fibroblasts to induced pluripotent stem cells. Nature 458 , 766–770 (2009).
Liu, Z. et al. Systematic comparison of 2A peptides for cloning multi-genes in a polycistronic vector. Sci. Rep. 7 , 2193 (2017).
Merkle, F. T. et al. Efficient CRISPR-Cas9-mediated generation of knockin human pluripotent stem cells lacking undesired mutations at the targeted locus. Cell Rep. 11 , 875–883 (2015).
Sakuma, T., Nakade, S., Sakane, Y., Suzuki, K. T. & Yamamoto, T. MMEJ-assisted gene knock-in using TALENs and CRISPR-Cas9 with the PITCh systems. Nat. Protoc. 11 , 118–133 (2016).
Gravina, A. et al. Synthetic immune checkpoint engagers protect HLA-deficient iPSCs and derivatives from innate immune cell cytotoxicity. Cell Stem Cell 30 , 1538–1548.e4 (2023).
Mo, F. et al. Engineered off-the-shelf therapeutic T cells resist host immune rejection. Nat. Biotechnol. 39 , 56–63 (2021).
Kochenderfer, J. N. et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119 , 2709–2720 (2012).
Mackensen, A. et al. Anti-CD19 CAR T cell therapy for refractory systemic lupus erythematosus. Nat. Med. 28 , 2124–2132 (2022).
Gomes-Silva, D. et al. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood 130 , 285–296 (2017).
Hu, Y. et al. Genetically modified CD7-targeting allogeneic CAR-T cell therapy with enhanced efficacy for relapsed/refractory CD7-positive hematological malignancies: a phase I clinical study. Cell Res. 32 , 995–1007 (2022).
Wellhausen, N. et al. Epitope base editing CD45 in hematopoietic cells enables universal blood cancer immune therapy. Sci. Transl. Med. 15 , eadi1145 (2023).
Mailankody, S. et al. Allogeneic BCMA-targeting CAR T cells in relapsed/refractory multiple myeloma: phase 1 UNIVERSAL trial interim results. Nat. Med. 29 , 422–429 (2023).
Chiesa, R. et al. Base-edited CAR7 T cells for relapsed T-cell acute lymphoblastic leukemia. N. Engl. J. Med. 389 , 899–910 (2023).
Loupy, A. & Lefaucheur, C. Antibody-mediated rejection of solid-organ allografts. N. Engl. J. Med. 379 , 1150–1160 (2018).
Wagner, D. L. et al. Immunogenicity of CAR T cells in cancer therapy. Nat. Rev. Clin. Oncol. 18 , 379–393 (2021).
Peraro, L. et al. Incorporation of bacterial immunoevasins to protect cell therapies from host antibody-mediated immune rejection. Mol. Ther. 29 , 3398–3409 (2021).
Gravina, A. et al. Protection of cell therapeutics from antibody-mediated killing by CD64 overexpression. Nat. Biotechnol. 41 , 717–727 (2023).
Fraietta, J. A. et al. Determinants of response and resistance to CD19 chimeric antigen receptor (CAR) T cell therapy of chronic lymphocytic leukemia. Nat. Med. 24 , 563–571 (2018).
Melenhorst, J. J. et al. Decade-long leukaemia remissions with persistence of CD4 + CAR T cells. Nature 602 , 503–509 (2022).
Forbes, S. J. & Rosenthal, N. Preparing the ground for tissue regeneration: from mechanism to therapy. Nat. Med. 20 , 857–869 (2014).
Galleu, A. et al. Apoptosis in mesenchymal stromal cells induces in vivo recipient-mediated immunomodulation. Sci. Transl. Med. 9 , eaam7828 (2017).
Yamasaki, S. et al. Low immunogenicity and immunosuppressive properties of human ESC- and iPSC-derived retinas. Stem Cell Rep. 16 , 851–867 (2021).
Abe, K. et al. Engraftment of allogeneic iPS cell-derived cartilage organoid in a primate model of articular cartilage defect. Nat. Commun. 14 , 804 (2023).
Mendicino, M., Bailey, A. M., Wonnacott, K., Puri, R. K. & Bauer, S. R. MSC-based product characterization for clinical trials: an FDA perspective. Cell Stem Cell 14 , 141–145 (2014).
Candinas, D. et al. Sex mismatch as a risk factor for chronic rejection of liver allografts. Lancet 346 , 1117–1121 (1995).
Gratwohl, A., Döhler, B., Stern, M. & Opelz, G. H-Y as a minor histocompatibility antigen in kidney transplantation: a retrospective cohort study. Lancet 372 , 49–53 (2008).
Wright, D. J. et al. Genetic variants associated with mosaic Y chromosome loss highlight cell cycle genes and overlap with cancer susceptibility. Nat. Genet. 49 , 674–679 (2017).
Nazor, K. L. et al. Recurrent variations in DNA methylation in human pluripotent stem cells and their differentiated derivatives. Cell Stem Cell 10 , 620–634 (2012).
Mekhoubad, S. et al. Erosion of dosage compensation impacts human iPSC disease modeling. Cell Stem Cell 10 , 595–609 (2012).
Hawksworth, J. et al. Enhancement of red blood cell transfusion compatibility using CRISPR-mediated erythroblast gene editing. EMBO Mol. Med. 10 , e8454 (2018).
Hildebrandt, M. R. et al. Precision health resource of control iPSC lines for versatile multilineage differentiation. Stem Cell Rep. 13 , 1126–1141 (2019).
Lombardo, A. et al. Gene editing in human stem cells using zinc finger nucleases and integrase-defective lentiviral vector delivery. Nat. Biotechnol. 25 , 1298–1306 (2007).
Cermak, T. et al. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 39 , e82 (2011).
Jinek, M. et al. A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337 , 816–821 (2012).
Zetsche, B. et al. Cpf1 is a single RNA-guided endonuclease of a class 2 CRISPR-Cas system. Cell 163 , 759–771 (2015).
Strecker, J. et al. Engineering of CRISPR-Cas12b for human genome editing. Nat. Commun. 10 , 212 (2019).
Morisaka, H. et al. CRISPR-Cas3 induces broad and unidirectional genome editing in human cells. Nat. Commun. 10 , 5302 (2019).
Paquet, D. et al. Efficient introduction of specific homozygous and heterozygous mutations using CRISPR/Cas9. Nature 533 , 125–129 (2016).
Ishida, K. et al. Site-specific randomization of the endogenous genome by a regulatable CRISPR-Cas9 piggyBac system in human cells. Sci. Rep. 8 , 310 (2018).
Martin, R. M. et al. Highly efficient and marker-free genome editing of human pluripotent stem cells by CRISPR-Cas9 RNP and AAV6 donor-mediated homologous recombination. Cell Stem Cell 24 , 821–828.e5 (2019).
Maurissen, T. L. & Woltjen, K. Synergistic gene editing in human iPS cells via cell cycle and DNA repair modulation. Nat. Commun. 11 , 2876 (2020).
Bodai, Z., Bishop, A. L., Gantz, V. M. & Komor, A. C. Targeting double-strand break indel byproducts with secondary guide RNAs improves Cas9 HDR-mediated genome editing efficiencies. Nat. Commun. 13 , 2351 (2022).
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533 , 420–424 (2016).
Gaudelli, N. M. et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551 , 464–471 (2017).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576 , 149–157 (2019).
Raguram, A., Banskota, S. & Liu, D. R. Therapeutic in vivo delivery of gene editing agents. Cell 185 , 2806–2827 (2022).
Taha, E. A., Lee, J. & Hotta, A. Delivery of CRISPR-Cas tools for in vivo genome editing therapy: trends and challenges. J. Control. Release 342 , 345–361 (2022).
Wolff, J. H. & Mikkelsen, J. G. Delivering genes with human immunodeficiency virus-derived vehicles: still state-of-the-art after 25 years. J. Biomed. Sci. 29 , 79 (2022).
Canté-Barrett, K. et al. Lentiviral gene transfer into human and murine hematopoietic stem cells: size matters. BMC Res. Notes 9 , 312 (2016).
Mitchell, R. S. et al. Retroviral DNA integration: ASLV, HIV, and MLV show distinct target site preferences. PLoS Biol. 2 , E234 (2004).
Wang, G. P., Ciuffi, A., Leipzig, J., Berry, C. C. & Bushman, F. D. HIV integration site selection: analysis by massively parallel pyrosequencing reveals association with epigenetic modifications. Genome Res. 17 , 1186–1194 (2007).
Wu, S. C. et al. piggyBac is a flexible and highly active transposon as compared to Sleeping Beauty , Tol2 , and Mos1 in mammalian cells. Proc. Natl Acad. Sci. USA 103 , 15008–15013 (2006).
Mitra, R., Fain-Thornton, J. & Craig, N. L. piggyBac can bypass DNA synthesis during cut and paste transposition. EMBO J. 27 , 1097–1109 (2008).
Nakanishi, H., Higuchi, Y., Kawakami, S., Yamashita, F. & Hashida, M. piggyBac transposon-mediated long-term gene expression in mice. Mol. Ther. 18 , 707–714 (2010).
Horie, K. et al. Efficient chromosomal transposition of a Tc1 / mariner -like transposon Sleeping Beauty in mice. Proc. Natl Acad. Sci. USA 98 , 9191–9196 (2001).
Fischer, S. E., Wienholds, E. & Plasterk, R. H. Regulated transposition of a fish transposon in the mouse germ line. Proc. Natl Acad. Sci. USA 98 , 6759–6764 (2001).
Yoshida, J. et al. Chromatin states shape insertion profiles of the piggyBac, Tol2 and Sleeping Beauty transposons and murine leukemia virus. Sci. Rep. 7 , 43613 (2017).
Huang, X. et al. Gene transfer efficiency and genome-wide integration profiling of Sleeping Beauty , Tol2 , and piggyBac transposons in human primary T cells. Mol. Ther. 18 , 1803–1813 (2010).
Liew, C. G., Draper, J. S., Walsh, J., Moore, H. & Andrews, P. W. Transient and stable transgene expression in human embryonic stem cells. Stem Cell s 25 , 1521–1528 (2007).
Hotta, A. & Ellis, J. Retroviral vector silencing during iPS cell induction: an epigenetic beacon that signals distinct pluripotent states. J. Cell. Biochem. 105 , 940–948 (2008).
Sasu, B. J. et al. Detection of chromosomal alteration after infusion of gene-edited allogeneic CAR T cells. Mol. Ther. 31 , 676–685 (2023).
Zhao, Y., Stepto, H. & Schneider, C. K. Development of the first World Health Organization lentiviral vector standard: toward the production control and standardization of lentivirus-based gene therapy products. Hum. Gene Ther. Methods 28 , 205–214 (2017).
Howe, S. J. et al. Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients. J. Clin. Investig. 118 , 3143–3150 (2008).
Hacein-Bey-Abina, S. et al. Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1. J. Clin. Investig. 118 , 3132–3142 (2008).
Sugita, S. et al. Lack of T cell response to iPSC-derived retinal pigment epithelial cells from HLA homozygous donors. Stem Cell Rep. 7 , 619–634 (2016).
Brehm, M. A., Shultz, L. D., Luban, J. & Greiner, D. L. Overcoming current limitations in humanized mouse research. J. Infect. Dis. 208 , S125–S130 (2013).
Herndler-Brandstetter, D. et al. Humanized mouse model supports development, function, and tissue residency of human natural killer cells. Proc. Natl Acad. Sci. USA 114 , E9626–E9634 (2017).
Matsuda, M. et al. Human NK cell development in hIL-7 and hIL-15 knockin NOD/SCID/IL2rgKO mice. Life Sci. Alliance 2 , e201800195 (2019).
Katano, I. et al. Predominant development of mature and functional human NK cells in a novel human IL-2–producing transgenic NOG mouse. J. Immunol. 194 , 3513–3525 (2015).
Brehm, M. A. et al. Lack of acute xenogeneic graft-versus-host disease, but retention of T-cell function following engraftment of human peripheral blood mononuclear cells in NSG mice deficient in MHC class I and II expression. FASEB J. 33 , 3137–3151 (2019).
Morizane, A. et al. Direct comparison of autologous and allogeneic transplantation of iPSC-derived neural cells in the brain of a non-human primate. Stem Cell Rep. 1 , 283–292 (2013).
Bjornson-Hooper, Z. B. et al. A comprehensive atlas of immunological differences between humans, mice, and non-human primates. Front. Immunol. 13 , 867015 (2022).
Verdun, N. & Marks, P. Secondary cancers after chimeric antigen receptor T-cell therapy. N. Engl. J. Med. 390 , 584–586 (2024).
Foster, M. C. et al. Utility of a safety switch to abrogate CD19.CAR T-cell-associated neurotoxicity. Blood 137 , 3306–3309 (2021).
Nishimura, T. et al. Sufficiency for inducible Caspase-9 safety switch in human pluripotent stem cells and disease cells. Gene Ther. 27 , 525–534 (2019).
Harding, J., Vintersten-Nagy, K. & Nagy, A. Universal stem cells: making the unsafe safe. Cell Stem Cell 27 , 198–199 (2020).
Shi, Z. D., Tchao, J., Wu, L. & Carman, A. J. Precision installation of a highly efficient suicide gene safety switch in human induced pluripotent stem cells. Stem Cells Transl. Med. 9 , 1378–1388 (2020).
Liang, Q. et al. Linking a cell-division gene and a suicide gene to define and improve cell therapy safety. Nature 563 , 701–704 (2018).
Wiebking, V. et al. Metabolic engineering generates a transgene-free safety switch for cell therapy. Nat. Biotechnol. 38 , 1441–1450 (2020).
Introna, M. et al. Genetic modification of human T cells with CD20: a strategy to purify and lyse transduced cells with anti-CD20 antibodies. Hum. Gene Ther. 11 , 611–620 (2000).
Wang, X. et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 118 , 1255–1263 (2011).
Philip, B. et al. A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood 124 , 1277–1287 (2014).
de Luzy, I. R. et al. Human stem cells harboring a suicide gene improve the safety and standardisation of neural transplants in Parkinsonian rats. Nat. Commun. 12 , 3275 (2021).
Wang, D., Quan, Y., Yan, Q., Morales, J. E. & Wetsel, R. A. Targeted disruption of the β 2 -microglobulin gene minimizes the immunogenicity of human embryonic stem cells. Stem Cells Transl. Med. 4 , 1234–1245 (2015).
Yoshihara, E. et al. Immune-evasive human islet-like organoids ameliorate diabetes. Nature 586 , 606–611 (2020).
Sintov, E. et al. Whole-genome CRISPR screening identifies genetic manipulations to reduce immune rejection of stem cell-derived islets. Stem Cell Rep. 17 , 1976–1990 (2022).
Chimienti, R. et al. Engineering of immune checkpoints B7-H3 and CD155 enhances immune compatibility of MHC-I −/ − iPSCs for β cell replacement. Cell Rep. 40 , 111423 (2022).
Gerace, D. et al. Engineering human stem cell-derived islets to evade immune rejection and promote localized immune tolerance. Cell Rep. Med. 4 , 100879 (2023).
Griffith, B. P. et al. Genetically modified porcine-to-human cardiac xenotransplantation. N. Engl. J. Med. 387 , 35–44 (2022).
Locke, J. E., Kumar, V., Anderson, D. & Porrett, P. M. Normal graft function after pig-to-human kidney xenotransplant. JAMA Surg. 158 , 1106–1108 (2023).
Mohiuddin, M. M. et al. Graft dysfunction in compassionate use of genetically engineered pig-to-human cardiac xenotransplantation: a case report. Lancet 402 , 397–410 (2023).
Kamatani, T. et al. Evaluation of immunosuppression protocols for MHC-matched allogeneic iPS cell-based transplantation using a mouse skin transplantation model. Inflamm. Regen. 42 , 4 (2022).
Liu, E. et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 382 , 545–553 (2020).
Matheus, F., Raveh, T., Oro, A. E., Wernig, M. & Drukker, M. Is hypoimmunogenic stem cell therapy safe in times of pandemics? Stem Cell Rep. 17 , 711–714 (2022).
Morizane, A. et al. MHC matching improves engraftment of iPSC-derived neurons in non-human primates. Nat. Commun. 8 , 385 (2017).
Download references
Acknowledgements
The authors thank K. Hui and F. Nakamura for critically reading the manuscript. The authors’ research work is supported by the Japan Agency for Medical Research and Development (AMED) grant numbers JP22bm1123006 and JP23bm1323001 to A.H., and by the Canadian Institutes of Health Research (CIHR) Foundation grants, Canadian Research Chair, Ontario Research Fund and Medicine by Design (University of Toronto) funding to A.N.
Author information
Authors and affiliations.
Center for iPS Cell Research and Application (CiRA), Kyoto University, Kyoto, Japan
Akitsu Hotta
Sana Biotechnology, South San Francisco, CA, USA
Sonja Schrepfer
Lunenfeld–Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, Ontario, Canada
Andras Nagy
Australian Regenerative Medicine Institute, Monash University, Melbourne, Victoria, Australia
Department of Obstetrics and Gynaecology, University of Toronto, Toronto, Ontario, Canada
You can also search for this author in PubMed Google Scholar
Contributions
A.H. initiated the project. All authors contributed to all aspects of the article (researching data, writing, and reviewing and/or editing the manuscript).

Corresponding author
Correspondence to Akitsu Hotta .
Ethics declarations
Competing interests.
A.H. declares that he has acted as a scientific advisor for AstraZeneca, CiRA Foundation, Orizuru Therapeutics and Takeda Pharmaceuticals. S.S. declares that she is a scientific founder, employee and stockholder of Sana Biotechnology. A.N. declares that he is a co-founder of panCELLa.
Peer review
Peer review information.
Nature Reviews Bioengineering thanks Luis Hidalgo, Ken-ichiro Seino, Luhan Yang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
Gene Ontology: https://geneontology.org/
IPD-IMGT/HLA: https://www.ebi.ac.uk/ipd/imgt/hla/
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Reprints and permissions
About this article
Cite this article.
Hotta, A., Schrepfer, S. & Nagy, A. Genetically engineered hypoimmunogenic cell therapy. Nat Rev Bioeng (2024). https://doi.org/10.1038/s44222-024-00219-9
Download citation
Accepted : 17 June 2024
Published : 29 July 2024
DOI : https://doi.org/10.1038/s44222-024-00219-9
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing newsletter — what matters in science, free to your inbox daily.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
- My Bibliography
- Collections
- Citation manager
Save citation to file
Email citation, add to collections.
- Create a new collection
- Add to an existing collection
Add to My Bibliography
Your saved search, create a file for external citation management software, your rss feed.
- Search in PubMed
- Search in NLM Catalog
- Add to Search
RNA Interference based Midkine Gene Therapy for Hepatocellular Carcinoma
Affiliations.
- 1 Biochemistry and Molecular Biology Department, Theodor Bilharz Research Institute (TBRI), Giza, Egypt.
- 2 Faculty of Biotechnology, October University for Modern Sciences & Arts (MSA), Giza, Egypt.
- 3 Department of Pathology, Theodor Bilharz Research Institute, Cairo, Egypt.
- 4 Department of Hepatobiliopancreatic Surgery, National Hepatology and Tropical Medicine Research Institute, (NHTMRI), Cairo, Egypt.
- 5 Fellow of Biochemistry, Obstetrics and Gynecology Hospital, Ain Shams University, Cairo, Egypt.
- PMID: 39068570
- DOI: 10.31557/APJCP.2024.25.7.2371
Background: Hepatocellular carcinoma (HCC) arises from hepatocytes and accounts for 90% of primary liver cancer. Reasons for HCC prognosis remaining dismal are that HCC is asymptomatic in its early stages, leading to late diagnosis, and it is markedly resistant to conventional chemo- and radiotherapy. In this study, we investigated RNA interference (RNAi)-based treatment for HCC by targeting MDK.
Aim: The present study aimed to evaluate MDK serum levels as a diagnostic biomarker for HCC detection and the effect of MDK silencing by RNAi on HCC.
Subjects and methods: A total of 140 participants, including 120 patients diagnosed with HCC and 20 healthy volunteers were enrolled in this study, all patients who underwent liver resection were sampled for tumor and adjacent non-tumor liver tissues, in addition to 5 ml of blood sample. Midkine expression levels were evaluated by ELISA and by qRT-PCR. The in vitro transfection and gene knockdown efficiency of midkine by MDK-siRNA was detected by qRT-PCR and ELISA. Gene knockdown effect at the molecule level on the proliferation of HepG2 in vitro was determined by cell counting.
Results: The results showed that the expression of MDK was significantly increased in the serum of HCC patients compared to control serum samples with P<0.001 and significant elevated expression levels of MDK in tumor tissues compared to non-tumor ones with P<0.001. It also showed that down-regulation of MDK using RNAi can significantly inhibit HepG2 cells.
Conclusion: Molecular targeting of MDK using RNAi interference decreases proliferation and could be a therapeutic target.
Keywords: Gene Therapy; HCC; Midkine; RNAi.
PubMed Disclaimer
- Search in MeSH
Related information
Linkout - more resources, full text sources.
- Asian Pacific Organization for Cancer Prevention
- MedlinePlus Health Information

- Citation Manager
NCBI Literature Resources
MeSH PMC Bookshelf Disclaimer
The PubMed wordmark and PubMed logo are registered trademarks of the U.S. Department of Health and Human Services (HHS). Unauthorized use of these marks is strictly prohibited.
An official website of the United States government
The .gov means it’s official. Federal government websites often end in .gov or .mil. Before sharing sensitive information, make sure you’re on a federal government site.
The site is secure. The https:// ensures that you are connecting to the official website and that any information you provide is encrypted and transmitted securely.
- Publications
- Account settings
Preview improvements coming to the PMC website in October 2024. Learn More or Try it out now .
- Advanced Search
- Journal List
- Mol Ther Methods Clin Dev
Clinical development of gene therapy: results and lessons from recent successes
Sandeep rp kumar.
1 Department of Pediatrics and Powell Gene Therapy Center, University of Florida, Gainesville, Florida, USA
David M Markusic
Moanaro biswas, katherine a high.
2 Spark Therapeutics, Philadelphia, Pennsylvania, USA
Roland W Herzog
Therapeutic gene transfer holds the promise of providing lasting therapies and even cures for diseases that were previously untreatable or for which only temporary or suboptimal treatments were available. For some time, clinical gene therapy was characterized by some impressive but rare examples of successes and also several setbacks. However, effective and long-lasting treatments are now being reported from gene therapy trials at an increasing pace. Positive outcomes have been documented for a wide range of genetic diseases (including hematological, immunological, ocular, and neurodegenerative and metabolic disorders) and several types of cancer. Examples include restoration of vision in blind patients, eradication of blood cancers for which all other treatments had failed, correction of hemoglobinopathies and coagulation factor deficiencies, and restoration of the immune system in children born with primary immune deficiency. To date, about 2,000 clinical trials for various diseases have occurred or are in progress, and many more are in the pipeline. Multiple clinical studies reported successful treatments of pediatric patients. Design of gene therapy vectors and their clinical development are advancing rapidly. This article reviews some of the major successes in clinical gene therapy of recent years.
Gene therapy seeks to treat a disease by transferring one or more therapeutic nucleic acids to a patient’s cells or by correcting a defective gene, for example by gene editing. Hence, this technology has the potential to cure diseases that are treatable but not curable with conventional medications, and to provide treatments for diseases previously classified as untreatable. As with any new medical technology, translation of this concept initially led to a mixture of encouraging and disappointing results in clinical trials, and also some major setbacks. However, fueled by successful treatment of ocular diseases and primary immune deficiencies, the “comeback of gene therapy” was highlighted as one of the major scientific breakthroughs of the year by Science magazine in 2009 (refs. 1 , 2 ). Advances in the development of gene therapy vector systems, optimized for in vivo and ex vivo gene transfer, and increasing clinical experience with these technologies were major factors that have finally allowed medicine to capitalize on the potential of gene transfer for the treatment of human disease. As the field advanced gene therapy beyond correction of genetic disorders, the spectrum of applications vastly increased. In fact, eradication of blood cancers using chimeric antigen receptor (CAR)-modified T cells prompted Science magazine to select cancer immunotherapy as the biggest scientific breakthrough of 2013 (ref. 3 ).
Effective strategies for clinical gene therapy are based on either in vivo gene delivery to postmitotic target cells or tissues or ex vivo gene delivery into autologous cells followed by adoptive transfer back into the patient ( Figure 1 ). Among the various viral based vector systems, adeno-associated virus (AAV) vectors have demonstrated the greatest clinical success for in vivo gene delivery ( Figure 2 ). A wide array of serotypes and capsid variants enables the targeting of a variety of tissues and cell types. Clinical application of ex vivo gene therapy has primarily focused on gene delivery to autologous hematopoietic stem cells (HSC) to treat hematological and other disorders, or into differentiated lineages such as T lymphocytes for cancer immunotherapy. Retroviral vectors (γ-retroviral or lentivirus derived) are capable of integrating their therapeutic genetic payload into the target cells’ genome and have proven effective for hematopoietic cells. Early adverse events with γ-retroviral vectors have promoted a shift to the use of vectors based on lentivirus ( Figure 2 ), which show a better preclinical safety profile and more efficient gene delivery to nondividing cells. 4 , 5

In vivo versus ex vivo gene therapies for the treatment of genetic diseases and cancer. In vivo gene therapy involves direct introduction of vector (carrying the therapeutic gene) into the patient (either into or near the target organ). This strategy has achieved success in the treatment of eye diseases, neurological disorders, and hemophilia In ex vivo gene therapy, a patient’s cells ( e.g. , hematopoietic cells) are taken out of the body and then transduced by a vector in culture to incorporate the therapeutic gene. Finally, the gene-modified cells are transplanted back to the patient. Various inherited metabolic and immunological disorders and different types of cancers have been successfully treated with ex vivo gene therapy. AADC, aromatic L-amino acid decarboxylase; ADA-SCID, adenosine deaminase severe combined immunodeficiency; ALL, acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; LCA II, Leber’s congenital amaurosis II; LHON, Leber’s hereditary optic neuropathy; MLD, metachromatic leukodystrophy; SCID-X1, X-linked severe combined immunodeficiency; WAS, Wiskott-aldrich syndrome.

Schematic illustration of two viral vectors widely used in clinical gene therapy. ( a ) Adeno-associated viral (AAV) vectors are prevalently used for in vivo gene therapy. Given the many serotypes and capsid variants that have been developed, these vectors can target a wide variety of tissues but are limited by their transgene carrying capacity (~5 kb for single-stranded, ssAAV, and 2.5–3 kb for self-complementary, scAAV). ( b ) Lentiviral vectors (LV) can carry up to 8 kb of transgene and are used in many ex vivo gene therapy protocols, in particular for HSC gene transfer. LV can be pseudotyped with envelopes from different viruses and thereby adapted to a broad range of targets. cPPT, central polypurine tract; LTR, long terminal repeat; Ψ: Packaging signal; RRE, Rev responsive elements; SIN LTR, self-inactivating LTR (with partial deletion in U3 region of 3’LTR); WPRE, Woodchuck hepatitis viral post-transcriptional regulatory element.
Approval of the first-gene therapy product Glybera, an AAV vector for treatment of lipoprotein lipase deficiency, by the European Medicines Agency was an important first step in gene-based drug development. 6 Recent breakthroughs in clinical gene therapy trials have now emerged in a variety of monogenic diseases including primary immune deficiencies, hemoglobinopathies, hemophilia B, neurological diseases, ocular diseases, and cancer immunotherapies (excluding oncolytic cancer therapy, which is reviewed elsewhere). 7 This review aims to highlight recent successes in gene therapy clinical trials.
Trials and Tribulations on the Path of Treating Primary Immunodeficiencies
Primary immunodeficiencies (PIDs) are rare but life-threatening genetic diseases that severely compromise the integrity and functions of the immune system. Children born with these diseases are often referred to as “bubble boys” or “bubble girls”, as they have to live in a germ free environment because their immune system is unable to fight off microbes that are harmless to immune competent individuals. PIDs targeted by gene therapy include X-linked severe combined immunodeficiency (SCID-X1), adenosine deaminase–deficient severe combined immunodeficiency (ADA-SCID), chronic granulomatous disease, and Wiskott-Aldrich syndrome (WAS). These children typically suffer from recurrent infections, failure to thrive, and death in the first few years after birth (unless they undergo successful bone marrow transplantation). Patients with PID mostly rely on the availability of human leukocyte antigens (HLA)-matched donors for HSC transplantation. With only a small proportion of patients (<20%) finding compatible donors, alternate strategies to treat PIDs are highly desirable. 8 Growing success of various gene therapy protocols involving autologous HSCs opened up new treatment avenues for patients without a need for an HLA-matched donor while avoiding a need for immune suppression and the complication of graft versus host disease. 9–14
Early experience with gene therapy for SCID-X1, an immunodeficiency disorder characterized by the absence of T cells, impaired B-cell function, lack of natural killer (NK) cell development and γ-chain (γc) dependent cytokines, verified the concept that gene-corrected cells had a selective advantage and could therefore effectively reconstitute immune competence in treated patients. However, these trials also experienced a major setback, as use of murine γ-retroviral vector for ex vivo gene transfer led to the development of leukemia in 5 of the 20 patients treated, thus raising safety concerns regarding the use of γ-retroviral vectors. 15–18 Use of self-inactivating (SIN) viral vectors, devoid of long terminal repeats promoter/enhancer function, in recent gene therapy protocols has reduced the risk for insertional mutagenesis and clonal dominance. 19–21
A multicenter phase 1/2 clinical trial (# {"type":"clinical-trial","attrs":{"text":"NCT01410019","term_id":"NCT01410019"}} NCT01410019 , Paris; # {"type":"clinical-trial","attrs":{"text":"NCT01175239","term_id":"NCT01175239"}} NCT01175239 , London; and # {"type":"clinical-trial","attrs":{"text":"NCT01129544","term_id":"NCT01129544"}} NCT01129544 , United States) of SCID-X1 employed a SIN γ-retroviral vector to deliver a corrected copy of the interleukin-2 receptor γ chain ( IL2RG ) gene to autologous HSCs of nine patients. 22 Infusion of IL2RG gene transduced autologous HSCs into SCID-X1 patients restored the T-cell population in most patients, who were subsequently able to resolve pre-existing infections. During follow up of 1 to 3 years post-gene therapy, no adverse event related to insertional mutagenesis was reported. Although it is still early for assessment of long-term safety, these results clearly demonstrate the efficacy of these vectors in treating PIDs. Recent data also document that these gene therapy protocols result in faster immunological reconstitution after transplant than conventional haploidentical HSC transplant. 23
Murine γ-retroviral vectors have also been employed in gene therapy trials of ADA-SCID, a fatal primary immunodeficiency with impaired T-, B-, and NK-cell development, which puts patients at risk for severe opportunistic infections. 11 , 12 , 14 , 24 Patients with ADA-SCID have mutations in a gene-encoding adenosine deaminase (ADA), an enzyme responsible for clearance of toxic purine metabolites from the body. Initial gene therapy trials for ADA-SCID in the early 1990’s utilized γ-retroviral vectors carrying a corrected copy of ADA gene. 25 , 26 These trials were encouraging in that normalization of T lymphocyte counts in some of the treated patients was observed. However, overall results strongly reflected the need to improve engraftment and frequency of gene-corrected HSCs. Moreover, in these trials, the direct effect of gene transfer could not be ascertained as treated patients simultaneously received enzyme replacement therapy. In subsequent clinical trials, patients were preconditioned with a nonmyeloablative regimen, and enzyme replacement therapy was discontinued before infusing autologous HSCs transduced with γ-retroviral vector carrying a functional copy of ADA. 12 , 24 , 27 , 28 These measures could entirely reverse the disease phenotype. Follow-up studies in these patients confirmed gene correction in multiple cell lineages, leading to expression of normal ADA levels and restoration of immune competence. It is further encouraging that more than 40 ADA-SCID patients have been treated with these vectors without any signs of vector-related genotoxicity.
More recently, Otsu et al. 29 reported clinical findings of γ-retroviral vector mediated gene therapy for ADA-SCID in two patients. The study found that although systemic detoxification and immune recovery was only partial, both patients have not required enzyme replacement therapy for more than 5 years, with observations ongoing. Again, no adverse event related to vector integration was observed. However, due to safety concerns with γ-retroviral vectors, SIN lentiviral vectors (with cellular promoter and codon optimized ADA cDNA) are also being explored for ADA-SCID in clinical studies ( {"type":"clinical-trial","attrs":{"text":"NCT01380990","term_id":"NCT01380990"}} NCT01380990 , {"type":"clinical-trial","attrs":{"text":"NCT01852071","term_id":"NCT01852071"}} NCT01852071 ). 30 , 31 These studies employed a mild non-myeloablative conditioning with busulfan (5 mg/kg) before infusing gene-corrected autologous HSCs. Five patients have been treated so far, and significant improvements in total T-cell count and overall immune recovery have been observed at about 1 year of follow-up. These promising results emphasize that though γ-retroviral vectors appeared to be safe in ADA-SCID gene therapy, LVs represents a viable alternate for the future.
WAS is another PID, for which retroviral vector has been employed in clinical studies. During 2006–2009, 10 WAS patients were treated with autologous HSCs transduced retrovirally to carry a corrected copy of WASp gene (following mild myeloablation). 32 , 33 Gene-corrected HSCs engrafted well and showed proliferative and selective advantage over noncorrected cells. Therapeutics levels of WAS protein correlated with clinical benefits (partial to complete resolution of bleeding, eczema, immunodeficiency, and autoimmunity) in these patients. However, following 1–5 years of gene therapy, seven of these patients were reported to develop leukemia, of which two patients died. These clinical outcomes further emphasize the need to address potential risks and safety concerns associated with these vectors.
Based on the preclinical studies, SIN-LVs were developed for subsequent clinical trial for treatment of WAS (# {"type":"clinical-trial","attrs":{"text":"NCT01515462","term_id":"NCT01515462"}} NCT01515462 ). Results of this study support the safety and efficacy of these vectors. 10 In this trial, three children with WAS were treated with bone marrow derived autologous CD34 + HSCs, which were transduced ex vivo with LV-w1.6W vector carrying a corrected copy of the WASp gene. Gene-corrected cells were observed in bone marrow and peripheral blood of all three patients. Stable levels of WASp protein were observed. Most importantly, these patients showed considerable improvement in their clinical symptoms (such as eczema and recurrent infections) and experienced reduced disease severity as early as 6 months after gene therapy. In contrast to γ-retroviral gene therapy for WAS, no insertional mutagenesis or clonal dominance was observed in any of these patients after 20 to 32 months of follow-up. 34 Similar positive outcomes have recently been reported for additional patients, who now no longer require a germ-free environment or frequent hospitalization due to bleeding or infection. 35 , 36
Chronic granulomatous disease is a rare genetic disorder caused by mutation in the gp91phox subunit of nicotinamide adenine dinucleotide phosphate oxidase, leading to inability of phagocytic cells (mainly neutrophils and macrophages) to produce reactive oxygen species and therefore to clear bacterial and fungal infections efficiently. Initial clinical trials on gene therapy for chronic granulomatous disease proved relatively unsuccessful as it only provided transient clinical benefits to patients, mainly due to low engraftment of gene-corrected cells. 37 Moreover, use of γ-retroviral vectors resulted in clonal dominance of gene-corrected cells leading to the development of myelodysplastic syndrome and monosomy 7 in some of the patients. 31 , 37 , 38 Current research focuses on utilizing SIN LVs to avoid such insertional mutations and approaches such as the use of myeloid-specific promoter are being adapted to achieve better reconstitution of gene-corrected cells.
Progress Toward Treating Hemoglobin Disorders: β-Thalassemia and Sickle Cell Disease
Worldwide, the monogenic hemoglobin disorders sickle cell anemia and β-thalassemia are major causes of morbidity and early mortality. There are no ideal long-term treatments. Available therapies, while aiming to improve the quality and duration of life, are not curative and have side effects from long-term use. While bone marrow transplantation can be curative, a matched donor is required. Thus, these hematological diseases should be ideal targets for therapeutic gene transfer to HSC. However, since these diseases provide no selective survival advantage to the gene-corrected HSC, it has been a challenge to generate a sufficient number of gene-corrected HSC expressing appropriate levels of the corrected globin protein to correct the defect in erythrocytes. The common blood disorder β-thalassemia results from the loss of functional β-globin, an essential component of hemoglobin in erythrocytes. Current treatment for β-thalassemia patients is frequent blood transfusions and chelation therapy to prevent the accumulation of iron. The first success in correcting β-thalassemia was reported by Cavazzana-Calvo et al. 9 where they transduced autologous CD34 + HSCs with a SIN-LV encoding a functional copy of the β-globin gene followed by myeloablative conditioning prior to reinfusion of the gene-corrected HSCs. Since treatment, the subject has displayed stable hemoglobin levels and has been transfusion free for almost 2 years. Recently, early results were reported from 17 patients enrolled in phase 1/2 studies, in which LV transfer of an engineered β-globin gene to HSC was performed. Treatment was particularly successful in patients with some level of endogenous β-globin such as those with β E form. Here, expression of >2 g/dl largely eliminated the need for transfusions and iron chelation therapy, thus vastly improving the patients’ quality of life (even allowing these children to regularly attend school). 39 , 40 A more difficult situation occurs in patients with β 0 /β 0 genotype, as they lack endogenous expression entirely. Thus far, gene therapy more typically has resulted in only partial correction of the disease. These ongoing phase 1/2 trials for β-thalassemia (# {"type":"clinical-trial","attrs":{"text":"NCT01639690","term_id":"NCT01639690"}} NCT01639690 , # {"type":"clinical-trial","attrs":{"text":"NCT01745120","term_id":"NCT01745120"}} NCT01745120 , # {"type":"clinical-trial","attrs":{"text":"NCT02151526","term_id":"NCT02151526"}} NCT02151526 , and # {"type":"clinical-trial","attrs":{"text":"NCT02453477","term_id":"NCT02453477"}} NCT02453477 ) will provide important long-term follow-up data for the safety and efficacy of gene-corrected HSC and the stability of β-globin expression. With clinical studies being conducted in United States, Australia, Thailand, France, and Italy, a much more comprehensive assessment of the potential of gene therapy for globin disorders should be possible in the near future.
In patients with sickle cell disease, mutations in β-globin generate an abnormal form of hemoglobin, called hemoglobin S or sickle hemoglobin. As a result, red blood cells appear “sickle-shaped”, lack flexibility and stick to vessel walls. In addition to pain, blockage of blood vessels can occur that slows or stops the flow of blood, so that oxygen may not reach nearby tissues. LV-mediated HSC gene transfer of a β-globin sequence with a missense mutation that results in “anti-sickling” properties (β A-T87Q ) has now been successfully carried out in a 13-year-old patient with sickle cell disease who did not respond to treatment with hydroxyuria (which aims to increase fetal globin gene expression). 40 , 41 This subject has achieved 47% of β-globin expression derived from the therapeutic transgene and no longer requires red blood cell transfusions (>1-year follow-up). This outcome marks the beginning of a wider use of gene therapy in the treatment of globin disorders.
Toward a Cure for the Coagulation Disorder Hemophilia B
Hemophilia is a hematological disorder caused by mutations in the X-linked gene encoding coagulation factor VIII (hemophilia A) or IX (hemophilia B) and occurs in 1 in 5,000 or 1 in 30,000 male births worldwide, respectively. In its severe form (<1% coagulation activity), the resulting failure of the blood to clot causes spontaneous bleeds into joints and soft tissues, and can be life threatening. Hemophilia patients are currently treated with intravenous infusion of either recombinant or plasma derived FVIII or FIX proteins, which are infused up to two to three times per week in order to prevent serious internal bleeds. This life-long treatment is burdensome and expensive and, due to the costs of the protein drugs, is typically not available in third world countries. In contrast, gene therapy has the potential to be curative, lasting for many years after a single round of gene transfer (>10 years in canine models). Since there is no need for regulated gene expression, levels as low as 5% of normal have a significant impact on bleeding frequencies (FVIII and FIX are secreted in an inactive form with normal plasma levels of 200 and 5,000 ng/ml, respectively). FVIII is primarily synthesized in a subset of endothelial cells, including liver sinusoidal endothelial cells, while hepatocytes are the site of FIX synthesis. Nonetheless, design of a gene therapy for hemophilia offers many choices for target cells and tissues because a number of cell types are capable of synthesizing biologically active FVIII or FIX upon gene transfer.
In preclinical studies using both small and large animals with hemophilia, in vivo gene transfer to the liver using AAV vectors emerged as one of the most efficient and promising protocols. Hemophilia B is considered an ideal first target for this approach because FIX is more easily expressed at high levels than is the case for FVIII. Initial clinical trials by High and colleagues provided proof-of-principle that AAV2 gene transfer via the hepatic artery can correct hemophilia B (FIX deficiency). 42–44 A patient in the high-dose cohort of 2 × 10 12 vg/kg had expression of FIX for over 2 months, with activity levels peaking at ~12%. However, a cytotoxic T-cell response to the viral capsid prevented sustained therapeutic FIX expression, resulting in mild, vector-dose-dependent liver toxicity manifested as self-limited, asymptomatic elevation of transaminases. 43–45 Further studies revealed a memory CD8 + T cells against AAV capsid in humans (who are naturally infected with AAV) that likely eliminated transduced hepatocytes. 46 In addition, there is considerable prevalence of neutralizing antibodies against AAV (in particular against serotype 2) in the human population, which blocks gene transfer to the liver above a certain titer. While AAV vectors alone do not provoke strong immune responses unlike other viruses such as adenovirus, these results highlight that the immune system remains a hurdle for in vivo gene transfer.
Nathwani et al. initiated a second clinical trial using a self-complementary genome, a codon-optimized F9 sequence, a different AAV serotype (AAV8), with a reduced frequency of neutralizing antibodies, and a transient immune suppression (IS) regimen with prednisolone if patients presented with a loss in circulating FIX or mild transaminitis. 47 , 48 The switch to AAV8 also allowed for a less-invasive peripheral vein administration, and the lower incidence of neutralizing antibodies made the therapy available to more patients. Extensive testing in nonhuman primates supported the safety and efficacy of this vector in human clinical trials (# {"type":"clinical-trial","attrs":{"text":"NCT00979238","term_id":"NCT00979238"}} NCT00979238 ). 49 Ten hemophilia B patients lacking neutralizing antibodies to AAV8 were enrolled in escalating dose groups. A dose-dependent persistent expression of FIX (1 to 6% of normal levels) was observed in all participants after a single intravenous injection, allowing either the discontinuation of prophylactic FIX protein infusions or a significant reduction in frequency. 50 However, capsid-specific antibodies arose in all participants, which would likely block future readministration of vector. 51 Relatively high vector doses were required to lift the patients from severe disease (<1% of normal coagulation activity) to mild disease (>5% of normal). A dose of 2 × 10 12 vg/kg resulted in a rise of liver enzyme levels (aspartate aminotransferase and alanine aminotransferase) in four out of six participants. These patients were started on prednisolone at 60 mg/day, which was tapered and stopped over a period of ~8 weeks. While this regimen prevented the complete loss of circulating FIX protein, as seen in the initial AAV2 clinical trial, some patients had a reduction in circulating FIX levels from the early peak levels. However, it should be noted that all subjects experienced sustained multi-year expression of FIX. 52 , 53
A new phase 1/2 clinical trial was recently started by Monahan et al. in which they gained approval to use a naturally occurring hyperactive FIX variant (R338L, FIX-Padua) in a self-complementary AAV8 vector (# {"type":"clinical-trial","attrs":{"text":"NCT01687608","term_id":"NCT01687608"}} NCT01687608 ). 54–58 In preclinical studies using animal models of hemophilia B, long-term expression with improved catalytic activity of FIX variant (FIXR338L) at lower vector doses (scAAV8-FIXR338L) were observed. 59 Three dose cohorts (of up to 3 × 10 12 vg/kg) were incorporated. Early treatment data show that one patient receiving a mid-range dose of 1 × 10 12 vg/kg has achieved sustained levels of 20–25% of normal, which is considered curative. 60 , 61 However, subjects treated with the highest vector dose lost expression, showing transaminitis and IFN-γ producing T cells in response to viral capsid antigen. In contrast to the trial by Nathwani et al ., immune suppression with prednisolone could not rescue expression. Other patients of the mid-range dose group also achieved only transient therapeutic levels, though the reason for their loss of expression is unclear as no liver toxicity or T-cell responses were observed. On the positive side, with over 20 hemophilia B patients treated with different AAV- F9 vectors there has been no indication of patients developing an immune response against the FIX protein. Recent data from ongoing clinical trials suggest that stable therapeutic and even curative levels of FIX protein activity are now obtainable in patients. Going forward, several critical questions need to be answered. For example, why did one trial using scAAV8 consistently yield sustained levels >5% at the highest dose while a second trial with a similar vector and dose did not? Can this be explained by differences in the design ( e.g. , presence of immune stimulatory CpG motifs) or production of the vectors or by other factors? Why was immune suppression with steroid drugs successful in one trial but not the other? Could other immune suppression regimens and/or advancements in vector engineering provide superior results?
Advances in design and molecular evolution of AAV capsids and testing in humanized mouse models will hopefully result in vectors with better performance in the human liver, which will be critical in adapting this approach to hemophilia A, as expression and efficient secretion of the larger FVIII molecule is more challenging. 62–66 To what extent vectors with higher transduction efficiency allow for a reduction in vector dose in humans (which would reduce capsid antigen presentation) remains to be seen, as it is possible that a threshold in the form of a minimally required number of particles exists for efficient transduction. Similar efforts are ongoing to design protocols that overcome pre-existing immunity in humans and that limit capsid antigen presentation. 67–69 Superior immune suppression protocols are being developed in parallel in case vector development by itself is insufficient to solve the immunological hurdles. 49 , 70 , 71 With sufficient levels of hepatocyte transduction and transgene expression, AAV hepatic gene transfer mediated immunological tolerance induction may ultimately be used as a dual therapy to eliminate established inhibitory antibodies to coagulation factors and other therapeutic proteins and provide therapeutic protein expression. 72–75
Inherited Neurological Disorders
Neurological disorders are among the most difficult diseases to treat with conventional pharmacological drugs because of the complexity of the central nervous system (CNS) and the existence of physical barriers such as the blood brain barrier. Gene therapy can potentially overcome these limitations but also faces substantial hurdles to delivery of the vector, targeting specific cells types within the CNS, and having to achieve adequate levels of gene expression within a therapeutic window. Nonetheless, successful gene therapies have now been reported for various genetic diseases of the CNS such as adrenoleukodystrophy (ALD), metachromatic leukodystrophy, and aromatic L-amino acid decarboxylase (AADC) deficiency. Both integrating (LV) and nonintegrating (AAV) vectors have been successfully used in these gene therapy trails.
Successful use of LV in neurological disorders was first reported in ALD, a genetic disorder of CNS in which mutations in ABCD1 gene (encoding ALD enzyme) results in accumulation of very long chain fatty acids causing demyelination of CNS and the adrenal cortex. In this clinical study, two ALD patients (after a complete myeloablative conditioning) were treated with LV-mediated gene-corrected autologous HSCs. 76 More than 3 years of follow-up studies in these patients showed persistent therapeutic levels of ALD protein with no further demyelination of CNS and stabilization of disease. Moreover, no major safety concern was reported in any of these patients. 77 However, larger cohorts with longer follow-up periods are needed to strengthen the safety and efficacy profile of these vectors to promote their use in gene therapy of ALD and other neurological disorders.
Late infantile metachromatic leukodystrophy is a fatal genetic disorder, in which first sign of symptoms appear in the second year of life and patients die within the first decade of their life. These patients lack arylsulfatase A (ARSA), an enzyme whose deficiency leads to accumulation of sulfatide (a glycolipid with sulfate group) in myelin-producing cells, causing demyelination of the nervous system leading to severe motor and cognitive damage. Unfortunately, bone marrow transplant or HSC transplant are not effective treatments for this disease because replacement of resident tissue macrophages and microglia by the transplanted hematopoietic cell progeny does not keep pace with the rapidly progressing disease. While bone marrow transplant, if given early enough, may have some stabilizing effect on neurocognitive function, it typically fails to halt loss of motor function. Biffi et al. 13 (# {"type":"clinical-trial","attrs":{"text":"NCT01560182","term_id":"NCT01560182"}} NCT01560182 ) hypothesized that overexpression of ARSA in gene-modified hematopoietic cells might overcome these limitations of bone marrow transplant by delivering a level of ARSA that would correct neighboring cells and thus halt demyelination. The authors employed SIN-LV-mediated gene transfer in autologous CD34 + HSCs of three presymptomatic patients. A dose-adjusted treatment of busulfan prior to gene transfer resulted in engraftment of bone marrow and peripheral blood with high frequencies of gene-corrected cells. In this approach, microglia derived from gene-corrected HSC serve to deliver ARSA to the CNS. After 2 years of gene transfer, these children continued to produce therapeutic levels of functional ARSA enzyme and showed normal motor and cognitive development for their ages. Moreover, these patients are well past their expected age of disease manifestation. Insertional mutagenesis was not observed in these patients, although long-term results in these patients are still pending.
AADC deficiency is another devastating neuronal genetic disorder for which gene therapy is being explored, as existing drug therapy provides little to no benefit to the patients. AADC deficiency impairs the synthesis and secretion of neurotransmitters such as dopamine and serotonin leading to developmental delay, oculogyric crises, dystonia, truncal hypotonia, sweating, severe movement disorders, tongue protrusion, jaw spasms, and neurological impairment in infants. In a recent clinical trial (# {"type":"clinical-trial","attrs":{"text":"NCT01395641","term_id":"NCT01395641"}} NCT01395641 ) of AADC deficiency, Hwu et al. directly injected AAV2 vector carrying AADC gene into the bilateral putamen of four 4- to 6-year-old patients. 78 , 79 All subjects were reported to gain weight after 3 to 6 months post-gene transfer and had better head control and emotional stability. Importantly, their Alberta Infant Motor Scale, Peabody Developmental Motor Scale, and Comprehensive Developmental Inventory for Infants and Toddlers scores were better after 15 to 24 months of gene therapy, indicating improvement in their motor and cognitive functions. The only adverse events observed were transient increases in dyskinesia in two patients and frequent episodes of apnea in one patient, which declined to normal by 10 months after gene transfer. These results demonstrate the potential of AAV-mediated gene therapy delivered by intracerebral infusion. However, long-term efficacy of treatment remains to be documented. For example, a phase 1 clinical trial (# {"type":"clinical-trial","attrs":{"text":"NCT00229736","term_id":"NCT00229736"}} NCT00229736 ) of Parkinson’s disease showed initial improvement post-AAV-mediated gene transfer but failed to achieve a lasting effect. 80 Nonetheless, multi-year transgene expression in the human brain was documented in this as well as in a trial on Canavan disease. 80 , 81 In evaluation of these approaches, one has to keep in mind that design of phase 1 clinical safety studies for neurodegenerative disease is difficult. In older patients with advanced disease, it may not be possible to sufficiently restore the damage to achieve a therapeutic effect or to target gene transfer to the ideal part of the CNS. However, there are also limitations to the gene delivery technology, resulting in transduction of too few neurons or other targeted cell types in the CNS. Early AAV2-based vectors could not spread from the injection site because of binding to extracellular matrix components. Spread from the injection site is much improved with use of alternative serotypes and capsid engineering. Nonetheless, the route of administration also needs to be optimized to achieve delivery to wider areas of the CNS. For example, infusion of the vector into the cisterna magna (for delivery into cerebrospinal fluid) or use of vectors that can cross the blood brain barrier is being explored to replace conventional intracranial injections. 82 , 83 Finally, promising results on correction of motor neurons in infant children have now been reported for a clinical trial on intravenous AAV9 delivery for spinal muscular atrophy type 1 (# {"type":"clinical-trial","attrs":{"text":"NCT02122952","term_id":"NCT02122952"}} NCT02122952 ; 2016 annual meeting of the American Society of Gene and Cell Therapy).
Inherited Retinal Diseases
AAV vectors are highly effective in ocular gene transfer and have therefore been extensively used in gene therapy protocols of various retinal diseases, including inherited forms of blindness for which no treatment existed. Leber’s congenital amaurosis type 2 (LCA2) is the first retinal hereditary disease that showed impressive clinical success with this type of gene therapy. 84–87 In LCA2 patients, mutations in RPE65 gene prevent the expression of retinal pigment epithelium 65 kilodalton protein (RPE65), thereby impairing the process of visual photo-transduction and thus severely limiting vision in these patients. Three clinical studies carried out independently by different centers demonstrated that a single subretinal injection of AAV2 vector carrying the therapeutic gene ( RPE65 ) improved vision in treated regions of the retina, resulting in improved vision that was stable for at least 3 years. 84–86 , 88–92 One protocol was successfully expanded to a trial in pediatric patients, demonstrating that early intervention vastly improved the potential for restoring vision. 93 Patients showed a 2 log or more unit increase in pupillary light responses, and an 8-year-old child gained light sensitivity to nearly that of age-matched normal-sighted individuals. This approach has now been further advanced to a phase 3 clinical trial. 94
Recent long-term evaluation of patients from two of the aforementioned clinical trials (# {"type":"clinical-trial","attrs":{"text":"NCT00481546","term_id":"NCT00481546"}} NCT00481546 and # {"type":"clinical-trial","attrs":{"text":"NCT00643747","term_id":"NCT00643747"}} NCT00643747 ) suggested a decline in retinal sensitivity, visual acuity, and functional gain over time, which however has not been observed in the third study. 95 , 96 Differences in vector design, final formulation, immunomodulatory regimens used (transient around vector administration), and surgical approach, may all contribute to the observed differences. For safety reasons, patients enrolled in these early trials had received gene transfer to only one eye. Although safety and efficacy data from these clinical trials supported subsequent treatment of the contralateral eye, there was concern that induction of immunity to the viral capsid or possibly the transgene product would not only limit therapy from a second injection, but may also affect the functionality gained by the first treatment. On the other hand, since the eye is considered immune privileged and relatively low vector doses are used, it is hypothesized that repeated gene transfer is possible without toxicity. To address these points, Bennett et al. 97 designed a protocol (# {"type":"clinical-trial","attrs":{"text":"NCT01208389","term_id":"NCT01208389"}} NCT01208389 ) to inject the contralateral eye of three patients with the identical AAV2 vector encoding the RPE65 gene. Within 3 months of the second gene transfer, patients showed gradually improved sensitivity to dim light, activation of the visual cortex on fMRI, and navigational skills using the recently injected eye without any adverse effect to their previously injected eye. These functional gains were more pronounced in younger patients suggesting that older patients with highly degenerated retinas might benefit less. The extent of retinal degeneration in LCA2 patients, as in many inherited retinal dystrophies, advances with age. Preclinical studies in murine and canine models have shown that gene augmentation could slow down the process of degeneration provided that the therapeutic intervention starts at an early, predegenerative stage of the disease. 98–100 More recently, Cideciyan et al. 101 found that though there was markedly improved visual functionality in LCA2 patients treated with gene therapy, this did not halt progression of retinal degeneration. However, these results may reflect suboptimal vector dose/gene transfer at a stage of retinal degeneration that could not be salvaged. Therefore, future clinical studies will be designed to better address both visual functionality and slowing down/halting the process of retinal degeneration. Approaches to halt other genetic causes of retinal degeneration are also being developed and show promise. 102
Success with the LCA2 gene therapy has generated interest in developing gene therapy for other retinal diseases. MacLaren et al. 103 reported successful initial results of a gene therapy trial (# {"type":"clinical-trial","attrs":{"text":"NCT01461213","term_id":"NCT01461213"}} NCT01461213 ) for choroidermia. This retinal genetic disease is due to a nonfunctional copy of the CHM gene, resulting in slow and progressive degeneration of the patient’s photoreceptors, choroid and retinal pigmented epithelium, and leading to complete blindness by middle age. A subretinal administration of the AAV2 vector carrying the CHM gene substantially improved vision in two patients and increased retinal sensitivity in four more patients after 6 months of gene therapy. Working toward a gene therapy for Leber hereditary optic neuropathy, Koilkonda et al. 104 demonstrated in a murine model of this disease that a single intravitreal injection of scAAV2 (with triple Y-F mutations in AAV2 capsid) carrying a wild-type human ND4 gene (a mitochondrial gene affecting complex I of electron transport chain) was able to arrest further deterioration of the optic nerve. Further, a significantly high rate of complex-I-dependent ATP synthesis was observed in eyes rescued with ND4 , suggesting correction of the impaired electron transport chain. Two gene therapy trials (# {"type":"clinical-trial","attrs":{"text":"NCT01267422","term_id":"NCT01267422"}} NCT01267422 and {"type":"clinical-trial","attrs":{"text":"NCT02161380","term_id":"NCT02161380"}} NCT02161380 ) utilizing AAV as a vector for Leber hereditary optic neuropathy gene therapy are currently enrolling patients.
Chimeric Antigen Receptor-Based Immunotherapy
Successful gene therapy is not limited to genetic diseases. Cancer immunotherapy based on genetic modification of autologous T cells has received much attention as it is highly effective at eradicating B-cell leukemias and lymphomas that are resistant to standard therapies in cancer patients. Autologous CD8 + T cells are engineered to recognize and kill cells bearing tumor-specific antigens through a CAR that combines the specificity of a monoclonal antibody with the proliferative and cytotoxic abilities of an activated CD8 + T cell. Antigen receptor and costimulatory molecule signaling is complexed with antibody-based antigen recognition, bypassing the need for HLA restriction, which is often downregulated in transformed cells, or the requirement for antigen presenting cells. CAR-modified autologous CD8 + T cells are generated by ex vivo gene transfer with LV, expanded and can be banked for repeated transfusions under well-established cGMP-compliant manufacturing processes. 105 Three generations of CARs have been developed with different combinations of signaling domains, with second- and third-generation CARs showing the greatest efficacy. 106 , 107 CARs have been successfully employed in clinical trials of modified T cells in patients with relapsed and refractory B-cell leukemias, B-cell lymphoma, chronic lymphocytic leukemia (CLL), and acute lymphoblastic leukemia (ALL).
In one of the three seminal studies published recently, Brentjens et al. 108 , at the Memorial Sloan Kettering Cancer Center reported on the safety of infusing a second-generation CAR that coupled the T-cell receptor ζ chain with the costimulatory CD28 molecule. This 19-28z CAR was transduced into autologous T cells of eight patients with CLL (# {"type":"clinical-trial","attrs":{"text":"NCT00466531","term_id":"NCT00466531"}} NCT00466531 ) and one patient with ALL (# {"type":"clinical-trial","attrs":{"text":"NCT01044069","term_id":"NCT01044069"}} NCT01044069 ), with or without prior conditioning therapy. Patients had poor genetic prognostic markers, including deletions in the p53 gene and exhibited advanced disease as evidenced by bulky lymphadenopathy. All patients tolerated the infusion well and did not develop cytokine release syndrome. Of the four CLL patients treated with cyclophosphamide before T-cell infusion, three patients exhibited either stable disease or a marked reduction of peripheral lymphadenopathy, whereas B-cell aplasia was observed in B-ALL patients. Rapid trafficking of 19-28z+ T cells to sites of CD19 + tumor and in vivo persistence of transplanted cells was observed. In another clinical study (# {"type":"clinical-trial","attrs":{"text":"NCT01029366","term_id":"NCT01029366"}} NCT01029366 ) at the University of Pennsylvania, CD19 targeting CAR T cells that contained a costimulatory domain from CD137 (4-1BB) and the T-cell receptor ζ chain (CTL019) showed potent non-cross-resistant clinical activity after infusion in three of three patients treated with advanced and refractory CLL. 109 , 110 High levels of expansion of CTL019 CAR T cells (>1,000-fold) were observed in all three patients, who continued to express functional CARs at high levels for at least 6 months. Moreover, a portion of these cells persisted as memory CAR + T cells and retained anti-CD19 effector functionality. Of the three patients treated, there were two complete responses and one partial response lasting greater than 8 months after CTL019 infusion. In a third study, Kochenderfer et al. 111 at the NIH reported a phase 1 clinical trial of CLL (# {"type":"clinical-trial","attrs":{"text":"NCT00924326","term_id":"NCT00924326"}} NCT00924326 ), in which patients received chemotherapy followed by an infusion of autologous anti CD19-CAR transduced T cells and a follow-up course of intravenous IL-2. Six of the eight treated patients showed remissions of their malignancies, and four of eight patients had long-term elimination of CD19 + B-lineage cells. Anti-CD19 CAR-transduced T cells could be detected in the blood of patients for up to 181 days after infusion. Elevations in serum levels of IFN-γ and TNF-α correlated with significant toxicity observed in the patients. The same group also reported on a phase 1/2 clinical trial (# {"type":"clinical-trial","attrs":{"text":"NCT01087294","term_id":"NCT01087294"}} NCT01087294 ) using allogenic donor hematopoietic stem cells, with 3 out of 10 patients achieving remission. Donor-derived anti-CD19 CAR T cells were detected in the blood of 8 of the 10 patients. Associated toxicities were transient, and none of the patients receiving donor derived T cells experienced graft versus host disease. 112
Given these encouraging responses in patients with CLL, researchers extended their findings to the more aggressive B-ALL, which also expresses the CD19 antigen. Long-term survival of adult patients with relapsed B-ALL is dependent upon achieving a complete remission induced through chemotherapy followed by allo-HSC therapy. Lee et al. 113 recently reported a phase 1 dose-escalation trial (# {"type":"clinical-trial","attrs":{"text":"NCT01593696","term_id":"NCT01593696"}} NCT01593696 ) that had enrolled 21 patients with relapsed or refractory B-ALL. The maximum tolerated dose was established, and a complete response was observed in 14 of the 21 patients (representing 66.7% complete response rate). Cytokine release syndrome was observed in 3 of the 21 patients but was controlled by treatment. All other toxicities observed were also reversible. Although CD19 CAR T-cell expansion was observed, long time persistence was not seen past day 68. In another clinical study (# {"type":"clinical-trial","attrs":{"text":"NCT01044069","term_id":"NCT01044069"}} NCT01044069 ), Brentjens et al. 114 also infused autologous 19-28z+ CAR T cells into five relapsed adult B-ALL subjects with persistent morphological disease or minimal residual disease after salvage chemotherapy. Rapid tumor eradication and complete remissions were observed in all patients, with one relapse 90 days into therapy. While the infused CAR T cells were otherwise well tolerated, significantly elevated cytokine levels required lympholytic steroid therapy. Patients were subsequently able to undergo allo-HSC therapy 1–4 months after 19-28z+ CAR T-cell infusion. In a larger cohort, an overall complete response of 88% was observed, which in some cases occurred at 2 weeks or sooner after treatment began, allowing most treated patients to transition to allo-HSC therapy. 115 Maude et al. 116 used CTL019 CAR T cells to treat 30 patients with relapsed and refractory ALL (# {"type":"clinical-trial","attrs":{"text":"NCT01626495","term_id":"NCT01626495"}} NCT01626495 and # {"type":"clinical-trial","attrs":{"text":"NCT01029366","term_id":"NCT01029366"}} NCT01029366 ). Complete remission was observed in 90% patients after the infusion of LV transduced autologous CTL019 cells. CTL019 cells proliferated in vivo and were detectable in the blood, bone marrow and cerebrospinal fluid of patients for up to 11 months. Here, all patients developed cytokine release syndrome, which was severe in eight patients but was resolved by treatment with the anti-IL-6 receptor antibody tocilizumab. In an unexpected outcome from a previous study by this group (with CTL019 T-cell infusion into two patients with ALL), a relapse occurred in one patient with blast cells that no longer expressed CD19. These findings highlight the need to develop CARs that recognize other tumor-associated antigens in B-cell leukemias and lymphomas. 117
While the data summarized above demonstrate significant remission of malignancy by CAR T-cell therapy in CLL and ALL patients, serious but manageable adverse events, including B-cell aplasia, tumor lysis syndrome and cytokine release syndrome, have also been reported. Approaches providing a controlled inflammatory response to tumor lysis such as determining optimal costimulatory signaling domains or suicide gene switches are being explored to enhance safety. Further identification of tumor-specific antigens and their use in CAR T-cell therapy would avoid the killing of nonmalignant cells, thereby providing better specificity. CAR T-cell doses and conditioning regimens have definitive influences on the outcome of therapy and thus need to also be further evaluated. Use of CARs in other cell types may provide an alternate strategy for patients, in which the use of T cells is not feasible. For example, CAR NK cells have been shown to be cytotoxic to B-cell leukemia, and transducing CARs into FoxP3 + regulatory CD4 + T cells (Treg) could provide specific immunosuppression for the treatment of autoimmune diseases. 118–120 Similarly, transfer of T-cell receptor (TCR) genes into Treg or fusion proteins into B cells could be used clinically for immune tolerance induction. 121 , 122 CAR gene transfer to T and NK cells also shows potential for generating immunity to human immunodeficiency virus. 123 Finally, CAR T-cell therapies are now being developed for solid tumors and other malignancies.
TCR Gene-Modified T Cells for Cancer Immunotherapy
Gene transfer of cloned TCRs isolated from tumor infiltrating T cells represents another approach for T-cell-based cancer immunotherapy, especially for tumor antigens not expressed on the cell surface. In TCR gene therapy, the patient’s T cells are again engineered ex vivo to redirect their specificity toward a particular tumor antigen. These tumor-specific engineered T cells are then reinfused back to the patient, where they recognize tumor antigen in the context of HLAs in the tumor microenvironment. Various clinical trials have employed genetically modified TCRs to treat a wide variety of cancers (synovial cell sarcoma, neuroblastoma, melanoma, and colorectal cancer) with long-term tumor regression. 124–129 However, due to high sensitivity, these engineered T cells can target normal cells expressing low levels of target antigen, which has lead to serious “on target-off tumor” toxicities in treated patients. 125 , 127 , 130 Cancer-testes antigens (CTAs), a group of tumor-associated antigens, provide an excellent alternative target for TCR gene therapy, since their expression is limited to male germ cells in the testis and in some cases in ovary, trophoblasts, or placenta. Moreover, various types of cancers (including lung, breast, ovary, bladder, and melanoma) share many CTAs, with their expression frequency ranging from 30–80%. Therefore, one type of CTA-specific TCR gene therapy could be used against several types of cancers. Several recent clinical studies (# {"type":"clinical-trial","attrs":{"text":"NCT01350401","term_id":"NCT01350401"}} NCT01350401 ; # {"type":"clinical-trial","attrs":{"text":"NCT01352286","term_id":"NCT01352286"}} NCT01352286 ; # {"type":"clinical-trial","attrs":{"text":"NCT01273181","term_id":"NCT01273181"}} NCT01273181 ) utilized affinity enhanced TCRs to target MAGE-A3 (one of the CTAs highly expressed in different types of cancers) in melanoma, myeloma, and metastatic cancer patients. 131 , 132 Five of the nine metastatic patients treated by Morgan et al. 132 responded positively to the treatment and underwent tumor regression. However, three of the nine treated patients developed serious neurotoxicity related to the treatment, resulting in two fatalities. Post-autopsy assays on the deceased patients’ brains suggested that expression of MAGE-A12 in human brain tissue might have contributed to the neurotoxicity. Similarly, two melanoma patients treated by Linette et al. 131 , died due to cardiac shock following MAGE-A3-specific T-cell infusion. Post-autopsy assays suggested that nonspecific recognition of an unrelated peptide derived from the striated muscle-specific protein titin led to severe myocardial damage in these patients. Though tumor regression in five patients treated by Morgan et al ., demonstrate efficacy of the approach, overall results from these two studies raise serious safety concerns for use of MAGE-A family members as the target for TCR gene therapy. Moreover, inability to predict such adverse events in preclinical studies of these targets clearly underscores the need for superior means to identify potential off-targets of engineered TCRs.
Encouragingly, a very recent clinical study (# {"type":"clinical-trial","attrs":{"text":"NCT01352286","term_id":"NCT01352286"}} NCT01352286 ) by Rapoport et al. 133 reported the safety and efficacy of NY-ESO c259 , a human-derived affinity-enhanced TCR that recognizes a peptide shared by two CTAs (NY-ESO-1- and LAGE-1), in 20 patients with multiple myeloma. Infusion of the engineered T cells did not cause adverse events. Moreover, the infused cells showed target-specific antitumor activity with significant proliferation and persistence, which was well correlated with progression free survival in 16 of the 20 treated patients up to 20 months. These results suggest that targeting multiple antigens increases the chance to achieve complete remission. Such advances would probably be able to target a larger cohort of patients with improved outcomes. Although outside the scope of this review, it should be pointed out that cancer immunotherapy of solid tumors using genetically engineered oncolytic viruses has had similar success. 134
Future Outlook: Precision Gene Therapy Through Gene Editing
Common characteristics shared by the success of ongoing clinical gene therapy trials are that: (i) disease is caused by the absence of a functional protein and conversely the presence of a mutated protein does not interfere with the therapy; (ii) regulated expression is not necessary due to suboptimal expression levels and the specific disease; and (iii) phenotypic correction with gene therapy can be achieved either directly through gene delivery to postmitotic tissues or indirectly through genetic modification of stem cells. Thus for the broader application of gene therapy to treat genetic-based diseases, the field needs to advance beyond gene addition strategies. One such path is the incorporation of genome-editing technology to either correct endogenous disease causing genes or to specifically target the integration of a therapeutic gene into a defined genetic locus. Such tools include zinc finger nucleases (ZFNs), transcription activator-like effector nucleases (TALENs), and clustered regularly interspaced short palindromic repeat (CRISPR)–associated systems (CRISPR–Cas). These engineered endonucleases can be programmed to specifically target and alter a DNA sequence by introducing a double-strand break and can therefore be employed to correct a disease-causing mutation with great efficiency, representing a sophisticated tool for precision medicine. Much of initial work has focused on establishing proof of principle and developing reagents and animal models for these gene-editing tools. ZFNs are the first to be investigated in clinical trial (# {"type":"clinical-trial","attrs":{"text":"NCT00842634","term_id":"NCT00842634"}} NCT00842634 ). Tebas et al. 135 infused 12 patients with autologous CD4 + T cells, in which the CCR5 gene, a coreceptor of human immunodeficiency virus, was inactivated by ZFNs. The study reports a significant increase in CD4 + T cells postinfusion and long-term persistence of CCR5-modified CD4 + T cells in peripheral blood and mucosal tissue. In parallel, TALENs and CRISPR-Cas system are undergoing preclinical development as potentially more versatile gene-editing tools. For instance, the CRISPR-Cas system was employed to study the effects of gene modifications in postmitotic neurons in the mouse brain or to correct a hereditary disease, Tyrosinemia, in a mouse model. 136 , 137 ZFN-mediated gene editing and a system for targeting without using nucleases have shown promise in correction of the F9 gene in hepatocytes of hemophilia B mice. 138 , 139 However, the current efficiency of gene editing may be subtherapeutic for certain diseases, where edited cells have no proliferative or survival advantage, and off-target double strand breaks may induce genotoxicity. Therefore, these gene-editing tools need further refinement before they can be safely and effectively used in the clinic. In addition, a guideline should be established on the ethical use of gene-editing tools such as for the editing of embryos to correct germ line mutations. 140
Conclusions
Clinical gene therapy has matured over the past decade, so that the field can now point to several impressive successes. These include a wide variety of diseases and modes of gene transfer. LV and AAV vectors have primarily been used in these trials, while other vector systems are expected to also further advance in their clinical applications. Lessons learned from successes as well as from problems and impediments encountered in recent trials will drive innovation of clinical gene therapy approaches. Next-generation protocols are already being developed, which will also help expand the spectrum of diseases that can be treated by gene therapy. For some of the most difficult targets such as muscular dystrophies and several of the lysosomal storage and neurological disorders, rapid success is less likely. Nonetheless, continuous progress is being made toward future treatments. Ultimately, gene therapy will become more precise with the incorporation of gene-editing tools, as has recently demonstrated in genome editing of HSC. 141 , 142
Dr. High is a co-founder, employee, and equity holder in Spark Therapeutics. She also holds issued patents related to AAV gene therapy. Dr. Herzog holds issued patents related to AAV gene therapy and has been receiving royalty payments from Spark Therapeutics.
- Naldini, L (2009). Medicine. A comeback for gene therapy . Science 326 : 805–806. [ PubMed ] [ Google Scholar ]
- Herzog, RW, Cao, O and Srivastava, A (2010). Two decades of clinical gene therapy–success is finally mounting . Discov Med 9 : 105–111. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Couzin-Frankel, J (2013). Breakthrough of the year 2013. Cancer immunotherapy . Science 342 : 1432–1433. [ PubMed ] [ Google Scholar ]
- Wang, X, Shin, SC, Chiang, AF, Khan, I, Pan, D, Rawlings, DJ et al. (2015). Intraosseous delivery of lentiviral vectors targeting factor VIII expression in platelets corrects murine hemophilia A . Mol Ther 23 : 617–626. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brown, BD (2015). A shot in the bone corrects a genetic disease . Mol Ther 23 : 614–615. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Ylä-Herttuala, S (2012). Endgame: glybera finally recommended for approval as the first gene therapy drug in the European union . Mol Ther 20 : 1831–1832. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kaufman, HL, Kohlhapp, FJ and Zloza, A (2015). Oncolytic viruses: a new class of immunotherapy drugs . Nat Rev Drug Discov 14 : 642–662. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Griffith, LM, Cowan, MJ, Notarangelo, LD, Kohn, DB, Puck, JM, Pai, SY et al. workshop participants. (2014). Primary Immune Deficiency Treatment Consortium (PIDTC) report . J Allergy Clin Immunol 133 : 335–347. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cavazzana-Calvo, M, Payen, E, Negre, O, Wang, G, Hehir, K, Fusil, F et al. (2010). Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia . Nature 467 : 318–322. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Aiuti, A, Biasco, L, Scaramuzza, S, Ferrua, F, Cicalese, MP, Baricordi, C et al. (2013). Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome . Science 341 : 1233151. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Aiuti, A, Cattaneo, F, Galimberti, S, Benninghoff, U, Cassani, B, Callegaro, L et al. (2009). Gene therapy for immunodeficiency due to adenosine deaminase deficiency . N Engl J Med 360 : 447–458. [ PubMed ] [ Google Scholar ]
- Aiuti, A, Slavin, S, Aker, M, Ficara, F, Deola, S, Mortellaro, A et al. (2002). Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning . Science 296 : 2410–2413. [ PubMed ] [ Google Scholar ]
- Biffi, A, Montini, E, Lorioli, L, Cesani, M, Fumagalli, F, Plati, T et al. (2013). Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy . Science 341 : 1233158. [ PubMed ] [ Google Scholar ]
- Gaspar, HB, Cooray, S, Gilmour, KC, Parsley, KL, Zhang, F, Adams, S et al. (2011). Hematopoietic stem cell gene therapy for adenosine deaminase-deficient severe combined immunodeficiency leads to long-term immunological recovery and metabolic correction . Sci Transl Med 3 : 97ra80. [ PubMed ] [ Google Scholar ]
- Hacein-Bey-Abina, S, Garrigue, A, Wang, GP, Soulier, J, Lim, A, Morillon, E et al. (2008). Insertional oncogenesis in 4 patients after retrovirus-mediated gene therapy of SCID-X1 . J Clin Invest 118 : 3132–3142. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hacein-Bey-Abina, S, von Kalle, C, Schmidt, M, Le Deist, F, Wulffraat, N, McIntyre, E et al. (2003). A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency . N Engl J Med 348 : 255–256. [ PubMed ] [ Google Scholar ]
- Hacein-Bey-Abina, S, Von Kalle, C, Schmidt, M, McCormack, MP, Wulffraat, N, Leboulch, P et al. (2003). LMO2-associated clonal T cell proliferation in two patients after gene therapy for SCID-X1 . Science 302 : 415–419. [ PubMed ] [ Google Scholar ]
- Howe, SJ, Mansour, MR, Schwarzwaelder, K, Bartholomae, C, Hubank, M, Kempski, H et al. (2008). Insertional mutagenesis combined with acquired somatic mutations causes leukemogenesis following gene therapy of SCID-X1 patients . J Clin Invest 118 : 3143–3150. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Montini, E, Cesana, D, Schmidt, M, Sanvito, F, Ponzoni, M, Bartholomae, C et al. (2006). Hematopoietic stem cell gene transfer in a tumor-prone mouse model uncovers low genotoxicity of lentiviral vector integration . Nat Biotechnol 24 : 687–696. [ PubMed ] [ Google Scholar ]
- Modlich, U, Navarro, S, Zychlinski, D, Maetzig, T, Knoess, S, Brugman, MH et al. (2009). Insertional transformation of hematopoietic cells by self-inactivating lentiviral and gammaretroviral vectors . Mol Ther 17 : 1919–1928. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Xu, W, Russ, JL and Eiden, MV (2012). Evaluation of residual promoter activity in γ-retroviral self-inactivating (SIN) vectors . Mol Ther 20 : 84–90. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hacein-Bey-Abina, S, Pai, SY, Gaspar, HB, Armant, M, Berry, CC, Blanche, S et al. (2014). A modified γ-retrovirus vector for X-linked severe combined immunodeficiency . N Engl J Med 371 : 1407–1417. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Touzot, F, Moshous, D, Creidy, R, Neven, B, Frange, P, Cros, G et al. (2015). Faster T-cell development following gene therapy compared with haploidentical HSCT in the treatment of SCID-X1 . Blood 125 : 3563–3569. [ PubMed ] [ Google Scholar ]
- Candotti, F, Shaw, KL, Muul, L, Carbonaro, D, Sokolic, R, Choi, C et al. (2012). Gene therapy for adenosine deaminase-deficient severe combined immune deficiency: clinical comparison of retroviral vectors and treatment plans . Blood 120 : 3635–3646. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kohn, DB, Hershfield, MS, Carbonaro, D, Shigeoka, A, Brooks, J, Smogorzewska, EM et al. (1998). T lymphocytes with a normal ADA gene accumulate after transplantation of transduced autologous umbilical cord blood CD34+ cells in ADA-deficient SCID neonates . Nat Med 4 : 775–780. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kohn, DB, Weinberg, KI, Nolta, JA, Heiss, LN, Lenarsky, C, Crooks, GM et al. (1995). Engraftment of gene-modified umbilical cord blood cells in neonates with adenosine deaminase deficiency . Nat Med 1 : 1017–1023. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Aiuti, A, Cassani, B, Andolfi, G, Mirolo, M, Biasco, L, Recchia, A et al. (2007). Multilineage hematopoietic reconstitution without clonal selection in ADA-SCID patients treated with stem cell gene therapy . J Clin Invest 117 : 2233–2240. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gaspar, HB (2012). Gene therapy for ADA-SCID: defining the factors for successful outcome . Blood 120 : 3628–3629. [ PubMed ] [ Google Scholar ]
- Otsu, M, Yamada, M, Nakajima, S, Kida, M, Maeyama, Y, Hatano, N et al. (2015). Outcomes in two Japanese adenosine deaminase-deficiency patients treated by stem cell gene therapy with no cytoreductive conditioning . J Clin Immunol 35 : 384–398. [ PubMed ] [ Google Scholar ]
- Gaspar, BB, Rivat, C, Himoudi, N, Gilmour, K, Booth, C and Xu-Bayford, J (2014). Immunological and metabolic correction after lentiviral vector mediated haematopoietic stem cell gene therapy for ADA deficiency . J Clin Immunol 34 : Suppl 2 :S167–S168. [ Google Scholar ]
- Cicalese, MP and Aiuti, A (2015). Clinical applications of gene therapy for primary immunodeficiencies . Hum Gene Ther 26 : 210–219. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Boztug, K, Schmidt, M, Schwarzer, A, Banerjee, PP, Díez, IA, Dewey, RA et al. (2010). Stem-cell gene therapy for the Wiskott-Aldrich syndrome . N Engl J Med 363 : 1918–1927. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Braun, CJ, Boztug, K, Paruzynski, A, Witzel, M, Schwarzer, A, Rothe, M et al. (2014). Gene therapy for Wiskott-Aldrich syndrome–long-term efficacy and genotoxicity . Sci Transl Med 6 : 227ra33. [ PubMed ] [ Google Scholar ]
- Qasim, W and Gennery, AR (2014). Gene therapy for primary immunodeficiencies: current status and future prospects . Drugs 74 : 963–969. [ PubMed ] [ Google Scholar ]
- Ferrua, FM, Galimberti, S, Scaramuzza, S, Giannelli, S, Pajno, R, Dionisio, F, et al. (2015). Safety and clinical benefit of lentiviral hematopoietic stem cell gene therapy for Wiskott-Aldrich syndrome . Blood 126 : 259. [ Google Scholar ]
- Chu, JI, Myriam Armant, L, Male, F, Dansereau, CH, MacKinnon, B, Burke, CJ, et al. (2015). Gene therapy using a self-inactivating lentiviral vector improves clinical and laboratory manifestations of Wiskott-Aldrich syndrome . Blood 126 : 260. [ Google Scholar ]
- Grez, M, Reichenbach, J, Schwäble, J, Seger, R, Dinauer, MC and Thrasher, AJ (2011). Gene therapy of chronic granulomatous disease: the engraftment dilemma . Mol Ther 19 : 28–35. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Stein, S, Ott, MG, Schultze-Strasser, S, Jauch, A, Burwinkel, B, Kinner, A et al. (2010). Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease . Nat Med 16 : 198–204. [ PubMed ] [ Google Scholar ]
- Walters, MC, Suradej Hongeng, J, Kwiatkowski, J, Schiller, GJ, Kletzel, M, Ho, PJ, et al. (2015). Update of results from the Northstar Study (HGB-204): a phase 1/2 study of gene therapy for beta-thalassemia major via transplantation of autologous hematopoietic stem cells transduced ex-vivo with a lentiviral beta AT87Q-globin vector (LentiGlobin BB305 Drug Product) . Blood 126 : 201. [ Google Scholar ]
- Cavazzana, M, Emmanuel Payen, J-A, Suarez, F, Beuzard, Y, Touzot, F, Cavallesco, R, et al. (2015). Outcomes of gene therapy for severe sickle disease and beta-thalassemia major via transplantation of autologous hematopoietic stem cells transduced ex vivo with a lentiviral beta AT87Q-globin vector . Blood 126 : 202. [ Google Scholar ]
- Malik, P (2016). Gene therapy for hemoglobinopathies: tremendous successes and remaining caveats . Mol Ther 24 : 668–670. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kay, MA, Manno, CS, Ragni, MV, Larson, PJ, Couto, LB, McClelland, A et al. (2000). Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector . Nat Genet 24 : 257–261. [ PubMed ] [ Google Scholar ]
- Manno, CS, Chew, AJ, Hutchison, S, Larson, PJ, Herzog, RW, Arruda, VR et al. (2003). AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B . Blood 101 : 2963–2972. [ PubMed ] [ Google Scholar ]
- Manno, CS, Pierce, GF, Arruda, VR, Glader, B, Ragni, M, Rasko, JJ et al. (2006). Successful transduction of liver in hemophilia by AAV-Factor IX and limitations imposed by the host immune response . Nat Med 12 : 342–347. [ PubMed ] [ Google Scholar ]
- Mingozzi, F, Maus, MV, Hui, DJ, Sabatino, DE, Murphy, SL, Rasko, JE et al. (2007). CD8(+) T-cell responses to adeno-associated virus capsid in humans . Nat Med 13 : 419–422. [ PubMed ] [ Google Scholar ]
- Hui, DJ, Podsakoff, GM, Pein, GC, Ivanciu, L, Camire, RM, Ertl, H, et al. High Etiena Basner-Tschakarjan (2015). AAV capsid CD8+ T cell epitopes are highly conserved across AAV serotypes . Mol Ther Methods Clin Dev 2 : 15029. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nathwani, AC, Gray, JT, McIntosh, J, Ng, CY, Zhou, J, Spence, Y et al. (2007). Safe and efficient transduction of the liver after peripheral vein infusion of self-complementary AAV vector results in stable therapeutic expression of human FIX in nonhuman primates . Blood 109 : 1414–1421. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nathwani, AC, Gray, JT, Ng, CY, Zhou, J, Spence, Y, Waddington, SN et al. (2006). Self-complementary adeno-associated virus vectors containing a novel liver-specific human factor IX expression cassette enable highly efficient transduction of murine and nonhuman primate liver . Blood 107 : 2653–2661. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nathwani, AC, Rosales, C, McIntosh, J, Rastegarlari, G, Nathwani, D, Raj, D et al. (2011). Long-term safety and efficacy following systemic administration of a self-complementary AAV vector encoding human FIX pseudotyped with serotype 5 and 8 capsid proteins . Mol Ther 19 : 876–885. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nathwani, AC, Reiss, UM, Tuddenham, EG, Rosales, C, Chowdary, P, McIntosh, J et al. (2014). Long-term safety and efficacy of factor IX gene therapy in hemophilia B . N Engl J Med 371 : 1994–2004. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rogers, GL and Herzog, RW (2015). Gene therapy for hemophilia . Front Biosci (Landmark Ed) 20 : 556–603. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Nathwani, AC, Tuddenham, EG, Rangarajan, S, Rosales, C, McIntosh, J, Linch, DC et al. (2011). Adenovirus-associated virus vector-mediated gene transfer in hemophilia B . N Engl J Med 365 : 2357–2365. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- VandenDriessche, T and Chuah, MK (2015). Moving forward toward a cure for hemophilia B . Mol Ther 23 : 809–811. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brunetti-Pierri, N, Grove, NC, Zuo, Y, Edwards, R, Palmer, D, Cerullo, V et al. (2009). Bioengineered factor IX molecules with increased catalytic activity improve the therapeutic index of gene therapy vectors for hemophilia B . Hum Gene Ther 20 : 479–485. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cantore, A, Nair, N, Della Valle, P, Di Matteo, M, Màtrai, J, Sanvito, F et al. (2012). Hyperfunctional coagulation factor IX improves the efficacy of gene therapy in hemophilic mice . Blood 120 : 4517–4520. [ PubMed ] [ Google Scholar ]
- Finn, JD, Nichols, TC, Svoronos, N, Merricks, EP, Bellenger, DA, Zhou, S et al. (2012). The efficacy and the risk of immunogenicity of FIX Padua (R338L) in hemophilia B dogs treated by AAV muscle gene therapy . Blood 120 : 4521–4523. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schuettrumpf, J, Herzog, RW, Schlachterman, A, Kaufhold, A, Stafford, DW and Arruda, VR (2005). Factor IX variants improve gene therapy efficacy for hemophilia B . Blood 105 : 2316–2323. [ PubMed ] [ Google Scholar ]
- Suwanmanee, T, Hu, G, Gui, T, Bartholomae, CC, Kutschera, I, von Kalle, C et al. (2014). Integration-deficient lentiviral vectors expressing codon-optimized R338L human FIX restore normal hemostasis in Hemophilia B mice . Mol Ther 22 : 567–574. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Monahan, PE, Sun, J, Gui, T, Hu, G, Hannah, WB, Wichlan, DG et al. (2015). Employing a gain-of-function factor IX variant R338L to advance the efficacy and safety of hemophilia B human gene therapy: preclinical evaluation supporting an ongoing adeno-associated virus clinical trial . Hum Gene Ther 26 : 69–81. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Monahan, P, Powell, JS, Konkle, BA, Josephson, NC, Escobar, M, McPhee, SJ, et al. (2015). Update on a phase 1/2 open-label trial of BAX335, an adeno-associated virus 8 (AAV8) vector-based gene therapy program for hemophilia B . J Thromb Haemost 13 : 87, 13 87. [ Google Scholar ]
- Herzog, RW (2015). Hemophilia gene therapy: caught between a cure and an immune response . Mol Ther 23 : 1411–1412. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Marsic, D, Govindasamy, L, Currlin, S, Markusic, DM, Tseng, YS, Herzog, RW et al. (2014). Vector design Tour de Force: integrating combinatorial and rational approaches to derive novel adeno-associated virus variants . Mol Ther 22 : 1900–1909. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bissig-Choisat, B, Wang, L, Legras, X, Saha, PK, Chen, L, Bell, P et al. (2015). Development and rescue of human familial hypercholesterolaemia in a xenograft mouse model . Nat Commun 6 : 7339. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lisowski, L, Dane, AP, Chu, K, Zhang, Y, Cunningham, SC, Wilson, EM et al. (2014). Selection and evaluation of clinically relevant AAV variants in a xenograft liver model . Nature 506 : 382–386. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schaffer, DV (2014). AAV shuffles to the liver: commentary on Lisowski et al. Mol Ther Methods Clin Dev 1 : 14006. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kotterman, MA and Schaffer, DV (2014). Engineering adeno-associated viruses for clinical gene therapy . Nat Rev Genet 15 : 445–451. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mingozzi, F, Anguela, XM, Pavani, G, Chen, Y, Davidson, RJ, Hui, DJ et al. (2013). Overcoming preexisting humoral immunity to AAV using capsid decoys . Sci Transl Med 5 : 194ra92. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hoffman, BE and Herzog, RW (2013). Covert warfare against the immune system: decoy capsids, stealth genomes, and suppressors . Mol Ther 21 : 1648–1650. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Martino, AT, Basner-Tschakarjan, E, Markusic, DM, Finn, JD, Hinderer, C, Zhou, S et al. (2013). Engineered AAV vector minimizes in vivo targeting of transduced hepatocytes by capsid-specific CD8+ T cells . Blood 121 : 2224–2233. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Corti, M, Elder, M, Falk, D, Lawson, L, Smith, B, Nayak, S et al. (2014). B-Cell Depletion is Protective Against Anti-AAV Capsid Immune Response: A Human Subject Case Study . Mol Ther Methods Clin Dev 1 : 14033. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sarkar, D, Biswas, M, Liao, G, Seay, HR, Perrin, GQ, Markusic, DM et al. (2014). Ex vivo expanded autologous polyclonal regulatory T cells suppress inhibitor formation in hemophilia . Mol Ther Methods Clin Dev 1 : 14030. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sack, BK, Herzog, RW, Terhorst, C and Markusic, DM (2014). Development of gene transfer for induction of antigen-specific tolerance . Mol Ther Methods Clin Dev 1 : 14013. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Markusic, DM, Hoffman, BE, Perrin, GQ, Nayak, S, Wang, X, LoDuca, PA et al. (2013). Effective gene therapy for haemophilic mice with pathogenic factor IX antibodies . EMBO Mol Med 5 : 1698–1709. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Crudele, JM, Finn, JD, Siner, JI, Martin, NB, Niemeyer, GP, Zhou, S et al. (2015). AAV liver expression of FIX-Padua prevents and eradicates FIX inhibitor without increasing thrombogenicity in hemophilia B dogs and mice . Blood 125 : 1553–1561. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Finn, JD, Ozelo, MC, Sabatino, DE, Franck, HW, Merricks, EP, Crudele, JM et al. (2010). Eradication of neutralizing antibodies to factor VIII in canine hemophilia A after liver gene therapy . Blood 116 : 5842–5848. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cartier, N, Hacein-Bey-Abina, S, Bartholomae, CC, Veres, G, Schmidt, M, Kutschera, I et al. (2009). Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy . Science 326 : 818–823. [ PubMed ] [ Google Scholar ]
- Cartier, N, Hacein-Bey-Abina, S, Bartholomae, CC, Bougnères, P, Schmidt, M, Kalle, CV et al. (2012). Lentiviral hematopoietic cell gene therapy for X-linked adrenoleukodystrophy . Methods Enzymol 507 : 187–198. [ PubMed ] [ Google Scholar ]
- Hwu, WL, Muramatsu, S, Tseng, SH, Tzen, KY, Lee, NC, Chien, YH et al. (2012). Gene therapy for aromatic L-amino acid decarboxylase deficiency . Sci Transl Med 4 : 134ra61. [ PubMed ] [ Google Scholar ]
- Lee, NC, Muramatsu, S, Chien, YH, Liu, WS, Wang, WH, Cheng, CH et al. (2015). Benefits of neuronal preferential systemic gene therapy for neurotransmitter deficiency . Mol Ther 23 : 1572–1581. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mittermeyer, G, Christine, CW, Rosenbluth, KH, Baker, SL, Starr, P, Larson, P et al. (2012). Long-term evaluation of a phase 1 study of AADC gene therapy for Parkinson’s disease . Hum Gene Ther 23 : 377–381. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Leone, P, Shera, D, McPhee, SW, Francis, JS, Kolodny, EH, Bilaniuk, LT et al. (2012). Long-term follow-up after gene therapy for canavan disease . Sci Transl Med 4 : 165ra163. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Samaranch, L, Salegio, EA, San Sebastian, W, Kells, AP, Bringas, JR, Forsayeth, J et al. (2013). Strong cortical and spinal cord transduction after AAV7 and AAV9 delivery into the cerebrospinal fluid of nonhuman primates . Hum Gene Ther 24 : 526–532. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Choudhury, SR, Hudry, E, Maguire, CA, Sena-Esteves, M, Breakefield, XO and Grandi, P (2016). Viral vectors for therapy of neurologic diseases . Neuropharmacology (epub ahead of print). [ PMC free article ] [ PubMed ]
- Bainbridge, JW, Smith, AJ, Barker, SS, Robbie, S, Henderson, R, Balaggan, K et al. (2008). Effect of gene therapy on visual function in Leber’s congenital amaurosis . N Engl J Med 358 : 2231–2239. [ PubMed ] [ Google Scholar ]
- Cideciyan, AV, Hauswirth, WW, Aleman, TS, Kaushal, S, Schwartz, SB, Boye, SL et al. (2009). Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year . Hum Gene Ther 20 : 999–1004. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Maguire, AM, Simonelli, F, Pierce, EA, Pugh, EN Jr, Mingozzi, F, Bennicelli, J et al. (2008). Safety and efficacy of gene transfer for Leber’s congenital amaurosis . N Engl J Med 358 : 2240–2248. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Boye, SE, Boye, SL, Lewin, AS and Hauswirth, WW (2013). A comprehensive review of retinal gene therapy . Mol Ther 21 : 509–519. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Cideciyan, AV, Aleman, TS, Boye, SL, Schwartz, SB, Kaushal, S, Roman, AJ et al. (2008). Human gene therapy for RPE65 isomerase deficiency activates the retinoid cycle of vision but with slow rod kinetics . Proc Natl Acad Sci USA 105 : 15112–15117. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Hauswirth, WW, Aleman, TS, Kaushal, S, Cideciyan, AV, Schwartz, SB, Wang, L et al. (2008). Treatment of leber congenital amaurosis due to RPE65 mutations by ocular subretinal injection of adeno-associated virus gene vector: short-term results of a phase I trial . Hum Gene Ther 19 : 979–990. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Jacobson, SG, Cideciyan, AV, Ratnakaram, R, Heon, E, Schwartz, SB, Roman, AJ et al. (2012). Gene therapy for leber congenital amaurosis caused by RPE65 mutations: safety and efficacy in 15 children and adults followed up to 3 years . Arch Ophthalmol 130 : 9–24. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Simonelli, F, Maguire, AM, Testa, F, Pierce, EA, Mingozzi, F, Bennicelli, JL et al. (2010). Gene therapy for Leber’s congenital amaurosis is safe and effective through 1.5 years after vector administration . Mol Ther 18 : 643–650. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Testa, F, Maguire, AM, Rossi, S, Pierce, EA, Melillo, P, Marshall, K et al. (2013). Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital Amaurosis type 2 . Ophthalmology 120 : 1283–1291. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Maguire, AM, High, KA, Auricchio, A, Wright, JF, Pierce, EA, Testa, F et al. (2009). Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial . Lancet 374 : 1597–1605. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Schimmer, J and Breazzano, S (2015). Investor Outlook: Significance of the Positive LCA2 Gene Therapy Phase III Results . Hum Gene Ther Clin Dev 26 : 208–210. [ PubMed ] [ Google Scholar ]
- Jacobson, SG, Cideciyan, AV, Roman, AJ, Sumaroka, A, Schwartz, SB, Heon, E et al. (2015). Improvement and decline in vision with gene therapy in childhood blindness . N Engl J Med 372 : 1920–1926. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bainbridge, JW, Mehat, MS, Sundaram, V, Robbie, SJ, Barker, SE, Ripamonti, C et al. (2015). Long-term effect of gene therapy on Leber’s congenital amaurosis . N Engl J Med 372 : 1887–1897. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Bennett, J, Ashtari, M, Wellman, J, Marshall, KA, Cyckowski, LL, Chung, DC et al. (2012). AAV2 gene therapy readministration in three adults with congenital blindness . Sci Transl Med 4 : 120ra15. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kostic, C, Crippa, SV, Pignat, V, Bemelmans, AP, Samardzija, M, Grimm, C et al. (2011). Gene therapy regenerates protein expression in cone photoreceptors in Rpe65(R91W/R91W) mice . PLoS One 6 : e16588. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Li, X, Li, W, Dai, X, Kong, F, Zheng, Q, Zhou, X et al. (2011). Gene therapy rescues cone structure and function in the 3-month-old rd12 mouse: a model for midcourse RPE65 leber congenital amaurosis . Invest Ophthalmol Vis Sci 52 : 7–15. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Mowat, FM, Breuwer, AR, Bartoe, JT, Annear, MJ, Zhang, Z, Smith, AJ et al. (2013). RPE65 gene therapy slows cone loss in Rpe65-deficient dogs . Gene Ther 20 : 545–555. [ PubMed ] [ Google Scholar ]
- Cideciyan, AV, Jacobson, SG, Beltran, WA, Sumaroka, A, Swider, M, Iwabe, S et al. (2013). Human retinal gene therapy for Leber congenital amaurosis shows advancing retinal degeneration despite enduring visual improvement . Proc Natl Acad Sci USA 110 : E517–E525. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shanab, AY, Mysona, BA, Matragoon, S and El-Remessy, AB (2015). Silencing p75(NTR) prevents proNGF-induced endothelial cell death and development of acellular capillaries in rat retina . Mol Ther Methods Clin Dev 2 : 15013. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- MacLaren, RE, Groppe, M, Barnard, AR, Cottriall, CL, Tolmachova, T, Seymour, L et al. (2014). Retinal gene therapy in patients with choroideremia: initial findings from a phase ½ clinical trial . Lancet 383 : 1129–1137. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Koilkonda, RD, Yu, H, Chou, TH, Feuer, WJ, Ruggeri, M, Porciatti, V et al. (2014). Safety and effects of the vector for the Leber hereditary optic neuropathy gene therapy clinical trial . JAMA Ophthalmol 132 : 409–420. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Gee, AP (2015). Manufacturing genetically modified T cells for clinical trials . Cancer Gene Ther 22 : 67–71. [ PubMed ] [ Google Scholar ]
- Carpenito, C, Milone, MC, Hassan, R, Simonet, JC, Lakhal, M, Suhoski, MM et al. (2009). Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains . Proc Natl Acad Sci USA 106 : 3360–3365. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Choi, BD, Suryadevara, CM, Gedeon, PC, Herndon, JE 2nd, Sanchez-Perez, L, Bigner, DD et al. (2014). Intracerebral delivery of a third generation EGFRvIII-specific chimeric antigen receptor is efficacious against human glioma . J Clin Neurosci 21 : 189–190. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brentjens, RJ, Rivière, I, Park, JH, Davila, ML, Wang, X, Stefanski, J et al. (2011). Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias . Blood 118 : 4817–4828. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kalos, M, Levine, BL, Porter, DL, Katz, S, Grupp, SA, Bagg, A et al. (2011). T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia . Sci Transl Med 3 : 95ra73. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Porter, DL, Levine, BL, Kalos, M, Bagg, A and June, CH (2011). Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia . N Engl J Med 365 : 725–733. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kochenderfer, JN, Dudley, ME, Feldman, SA, Wilson, WH, Spaner, DE, Maric, I et al. (2012). B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells . Blood 119 : 2709–2720. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Kochenderfer, JN, Dudley, ME, Carpenter, RO, Kassim, SH, Rose, JJ, Telford, WG et al. (2013). Donor-derived CD19-targeted T cells cause regression of malignancy persisting after allogeneic hematopoietic stem cell transplantation . Blood 122 : 4129–4139. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lee, DW, Kochenderfer, JN, Stetler-Stevenson, M, Cui, YK, Delbrook, C, Feldman, SA et al. (2015). T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial . Lancet 385 : 517–528. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Brentjens, RJ, Davila, ML, Riviere, I, Park, J, Wang, X, Cowell, LG et al. (2013). CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia . Sci Transl Med 5 : 177ra38. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Davila, ML, Riviere, I, Wang, X, Bartido, S, Park, J, Curran, K et al. (2014). Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia . Sci Transl Med 6 : 224ra25. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Maude, SL, Frey, N, Shaw, PA, Aplenc, R, Barrett, DM, Bunin, NJ et al. (2014). Chimeric antigen receptor T cells for sustained remissions in leukemia . N Engl J Med 371 : 1507–1517. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Grupp, SA, Kalos, M, Barrett, D, Aplenc, R, Porter, DL, Rheingold, SR et al. (2013). Chimeric antigen receptor-modified T cells for acute lymphoid leukemia . N Engl J Med 368 : 1509–1518. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Blat, D, Zigmond, E, Alteber, Z, Waks, T and Eshhar, Z (2014). Suppression of murine colitis and its associated cancer by carcinoembryonic antigen-specific regulatory T cells . Mol Ther 22 : 1018–1028. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Fransson, M, Piras, E, Burman, J, Nilsson, B, Essand, M, Lu, B et al. (2012). CAR/FoxP3-engineered T regulatory cells target the CNS and suppress EAE upon intranasal delivery . J Neuroinflammation 9 : 112. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Shimasaki, N, Fujisaki, H, Cho, D, Masselli, M, Lockey, T, Eldridge, P et al. (2012). A clinically adaptable method to enhance the cytotoxicity of natural killer cells against B-cell malignancies . Cytotherapy 14 : 830–840. [ PubMed ] [ Google Scholar ]
- Kim, YC, Zhang, AH, Su, Y, Rieder, SA, Rossi, RJ, Ettinger, RA et al. (2015). Engineered antigen-specific human regulatory T cells: immunosuppression of FVIII-specific T- and B-cell responses . Blood 125 : 1107–1115. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang, X, Moghimi, B, Zolotukhin, I, Morel, LM, Cao, O and Herzog, RW (2014). Immune tolerance induction to factor IX through B cell gene transfer: TLR9 signaling delineates between tolerogenic and immunogenic B cells . Mol Ther 22 : 1139–1150. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Zhen, A, Kamata, M, Rezek, V, Rick, J, Levin, B, Kasparian, S et al. (2015). HIV-specific immunity derived from chimeric antigen receptor-engineered stem cells . Mol Ther 23 : 1358–1367. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Duval, L, Schmidt, H, Kaltoft, K, Fode, K, Jensen, JJ, Sorensen, SM et al. (2006). Adoptive transfer of allogeneic cytotoxic T lymphocytes equipped with a HLA-A2 restricted MART-1 T-cell receptor: a phase I trial in metastatic melanoma . Clin Cancer Res 12 : 1229–1236. [ PubMed ] [ Google Scholar ]
- Johnson, LA, Morgan, RA, Dudley, ME, Cassard, L, Yang, JC, Hughes, MS et al. (2009). Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen . Blood 114 : 535–546. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Morgan, RA, Dudley, ME, Wunderlich, JR, Hughes, MS, Yang, JC, Sherry, RM et al. (2006). Cancer regression in patients after transfer of genetically engineered lymphocytes . Science 314 : 126–129. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Parkhurst, MR, Yang, JC, Langan, RC, Dudley, ME, Nathan, DA, Feldman, SA et al. (2011). T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis . Mol Ther 19 : 620–626. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Pule, MA, Savoldo, B, Myers, GD, Rossig, C, Russell, HV, Dotti, G et al. (2008). Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma . Nat Med 14 : 1264–1270. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Robbins, PF, Morgan, RA, Feldman, SA, Yang, JC, Sherry, RM, Dudley, ME et al. (2011). Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1 . J Clin Oncol 29 : 917–924. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Morgan, RA, Yang, JC, Kitano, M, Dudley, ME, Laurencot, CM and Rosenberg, SA (2010). Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2 . Mol Ther 18 : 843–851. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Linette, GP, Stadtmauer, EA, Maus, MV, Rapoport, AP, Levine, BL, Emery, L et al. (2013). Cardiovascular toxicity and titin cross-reactivity of affinity-enhanced T cells in myeloma and melanoma . Blood 122 : 863–871. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Morgan, RA, Chinnasamy, N, Abate-Daga, D, Gros, A, Robbins, PF, Zheng, Z et al. (2013). Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy . J Immunother 36 : 133–151. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Rapoport, AP, Stadtmauer, EA, Binder-Scholl, GK, Goloubeva, O, Vogl, DT, Lacey, SF et al. (2015). NY-ESO-1-specific TCR-engineered T cells mediate sustained antigen-specific antitumor effects in myeloma . Nat Med 21 : 914–921. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Lichty, BD, Breitbach, CJ, Stojdl, DF and Bell, JC (2014). Going viral with cancer immunotherapy . Nat Rev Cancer 14 : 559–567. [ PubMed ] [ Google Scholar ]
- Tebas, P, Stein, D, Tang, WW, Frank, I, Wang, SQ, Lee, G et al. (2014). Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV . N Engl J Med 370 : 901–910. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Swiech, L, Heidenreich, M, Banerjee, A, Habib, N, Li, Y, Trombetta, J et al. (2015). In vivo interrogation of gene function in the mammalian brain using CRISPR-Cas9 . Nat Biotechnol 33 : 102–106. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Yin, H, Xue, W, Chen, S, Bogorad, RL, Benedetti, E, Grompe, M et al. (2014). Genome editing with Cas9 in adult mice corrects a disease mutation and phenotype . Nat Biotechnol 32 : 551–553. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Barzel, A, Paulk, NK, Shi, Y, Huang, Y, Chu, K, Zhang, F et al. (2015). Promoterless gene targeting without nucleases ameliorates haemophilia B in mice . Nature 517 : 360–364. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Li, H, Haurigot, V, Doyon, Y, Li, T, Wong, SY, Bhagwat, AS et al. (2011). In vivo genome editing restores haemostasis in a mouse model of haemophilia . Nature 475 : 217–221. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Frederickson, R and Ylä-Herttuala, S (2015). Molecular therapy special issue on gene editing technologies and applications . Mol Ther 23 : 1279. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Wang, J, Exline, CM, DeClercq, JJ, Llewellyn, GN, Hayward, SB, Li, PW et al. (2015). Homology-driven genome editing in hematopoietic stem and progenitor cells using ZFN mRNA and AAV6 donors . Nat Biotechnol 33 : 1256–1263. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Sather, BD, Romano Ibarra, GS, Sommer, K, Curinga, G, Hale, M, Khan, IF et al. (2015). Efficient modification of CCR5 in primary human hematopoietic cells using a megaTAL nuclease and AAV donor template . Sci Transl Med 7 : 307ra156. [ PMC free article ] [ PubMed ] [ Google Scholar ]
- Health Tech
- Health Insurance
- Medical Devices
- Gene Therapy
- Neuroscience
- H5N1 Bird Flu
- Health Disparities
- Infectious Disease
- Mental Health
- Cardiovascular Disease
- Chronic Disease
- Alzheimer's
- Coercive Care
- The Obesity Revolution
- The War on Recovery
- Adam Feuerstein
- Matthew Herper
- Jennifer Adaeze Okwerekwu
- Ed Silverman
- CRISPR Tracker
- Breakthrough Device Tracker
- Generative AI Tracker
- Obesity Drug Tracker
- 2024 STAT Summit
- Wunderkinds Nomination
- STAT Madness
- STAT Brand Studio
Don't miss out
Subscribe to STAT+ today, for the best life sciences journalism in the industry
How Pfizer’s grand gene therapy ambitions crumbled
By Jason Mast July 26, 2024

A decade ago, Pfizer began investing heavily in gene therapy, bringing in experimental treatments for a range of genetic diseases, pumping $800 million into “state-of-the-art” manufacturing facilities and announcing its intention to become an “industry leader” that would deliver “one-time, transformative therapies” for rare diseases.
Today, it’s not clear how much the company has to show for it.
advertisement
On Wednesday, Pfizer announced data from its hemophilia A gene therapy. The results set up a potential approval, but questions about durability may deter most patients from getting it. A potentially curative hemophilia B gene therapy was approved in April but faces competition. A Duchenne muscular dystrophy treatment, the linchpin of the portfolio, failed to show any benefit in a large trial last month.
STAT+ Exclusive Story
Already have an account? Log in

This article is exclusive to STAT+ subscribers
Unlock this article — plus daily coverage and analysis of the pharma industry — by subscribing to stat+..
Totals $468 per year
for 3 months, then $39/month
Then $39/month
Savings start at 25%!
Annually per user
$300 Annually per user
Get unlimited access to award-winning journalism and exclusive events.
About the Author Reprints
General Assignment Reporter
Jason Mast is a general assignment reporter at STAT focused on the science behind new medicines and the systems and people that decide whether that science ever reaches patients.
drug development
gene editing
Pharmaceuticals
STAT encourages you to share your voice. We welcome your commentary, criticism, and expertise on our subscriber-only platform, STAT+ Connect
To submit a correction request, please visit our Contact Us page .

Recommended

Recommended Stories

STAT Plus: On Andrew Left and the government’s fraud case

STAT Plus: Physicians weigh in on potential impact of Trump’s ear wound: ‘It’s a matter of inches’

STAT Plus: What to know about Trump VP pick J.D. Vance’s health care views and investments

STAT Plus: Sarepta demanded Duchenne patient advocacy group censor video critical of the company

STAT Plus: Mount Sinai mounted aggressive campaign to stifle debate over revelations about its controversial brain research

IMAGES
VIDEO
COMMENTS
One new arrival to the gene therapy scene is being watched particularly closely: in vivo gene editing using a system called CRISPR, which has become one of the most promising gene therapies since ...
Gene therapy has made inroads against cancer, too. ... helped to develop the therapy and published the first successful results in a 2010 study for the treatment of lymphoma. ... In SMA's case, ...
Introduction. Gene therapy offers a novel approach to treating monogenic diseases that, rather than only treating symptoms, targets the root cause of a disease by introducing a vector coding for a gene that compensates for a mutated or absent gene. 1 The pathological consequences of a disease may be prevented or substantially delayed after only a single gene therapy treatment. 2, 3, 4 Because ...
Case Report . A boy with the β S ... et al. Update of results from the Northstar Study (HGB-204): a phase 1/2 study of gene therapy for beta-thalassemia major via transplantation of autologous ...
The phase 1-2 HGB-206 study is evaluating the efficacy and safety of LentiGlobin for sickle cell disease, and an unprespecified interim analysis of the results is now reported in the Journal. 8 ...
Seventeen-year-old Jesse Gelsinger had a genetic disease called ornithine transcarbamylase (OTC) deficiency. OTC deficiency prevents the body from breaking down ammonia, a metabolic waste product. In patients with this disease, the excessive buildup of ammonia often causes death soon after birth, unless the patient's diet is immediately ...
Gene Therapy - Successes and challenges in clinical gene therapy. ... Smith B, Nayak S, et al. B-cell depletion is protective against anti-AAV capsid immune response: a human subject case study ...
Documented in studies of gene therapy for X-linked SCID, 1,2 Wiskott-Aldrich ... as is the case for other classes of therapeutics. 76 The other development is the issuance in 2018 of six ...
Therefore, historically, gene therapy and the discovery of antibiotics and chemotherapy agents, or any new technology, need more clarifying preclinical studies. In the future, there is the promise of applying these techniques in several fields of Medicine and a greater percentage of clinical trials.
Gene therapy approaches for CF. Gene therapy offers great hope for the treatment of genetic diseases/disorders. By replacing the genetic mutation with a "correct version" of the CFTR gene, this method offers a potentially permanent cure. Indeed, since the discovery of the CF gene, many studies have attempted to correct the CFTR mutations
Explore the what's and why's of gene therapy research, includingan in-depth look at the genetic disorder cystic fibrosis and how gene therapy could potentially be used to treat it. ... Gene Therapy Case Study: Cystic Fibrosis. Funding. Supported by a Science Education Partnership Award (SEPA) Grant No. R25RR023288 from the National Center for ...
Gene therapy, i.e., any therapeutic approach involving the use of genetic material as a drug and more largely altering the transcription or translation of one or more genes, covers a wide range of innovative methods for treating diseases, including neurological disorders. Although they share common …
Gene therapy gene therapy. A new, experimental method of fighting disease by replacing a defective gene with a healthy gene. has not yet been fully successful in overcoming any genetic diseases, so any patients who take part in early trials of a possible new treatment - and their parents - are very brave.
Genetic Science Learning Center. Home; Gene Therapy; Gene Therapy Case Study: Cystic Fibrosis
Case Study: Gene Therapy for Enhancement Purposes. Dr. Anderson specializes in a particular type of gene therapy that targets Alzheimer’s Disease (AD).  Neural degeneration and synapse loss in the brain are characteristic of AD. Therefore, this gene therapy aims to protect neurons from degeneration and enhance the function of any ...
The gene therapy, called OTL-103 (etuvetidigene autotemcel) also originated in the laboratories of the San Raffaele-Telethon Institute in Milan, and was the subject of an industrial partnership with Orchard. ... By downloading this case study, you acknowledge that GlobalData may share your information with Cytiva Thematic and that your personal ...
Hematopoietic stem cell gene therapy (HSCGT) Hematopoietic stem cell gene therapy (HSCGT) for inherited blood disorders uses the patient's own (autologous) HSC that are gene corrected either by adding a normal copy of the inherited defective gene with an integrating vector or, more recently, editing the defective gene to restore its function.. Because HSC can be removed from the body by bone ...
Gene Therapy. CASE STUDY. Background and Problem. Allucent's client requested support to bring an AAV. gene therapy for a rare neurodegenerative disease. into the clinic for a Phase 1b study in patients. Dose. scaling from pharmacology and toxicology studies by brain volume alone for administration directly into the brain by MRI-Guided ...
Immunological barriers to haematopoietic stem cell gene therapy ... donor cells from depletion by knockout of the CD52 gene 131. However, in some studies, ... al. Case report: transplantation of ...
Gene Therapy Manufacturing . Through the collaboration, our CDMO team will provide FRF access to extensive cell and gene therapy expertise and established plasmid and AAV production platforms, generating materials required for the foundation's Phase I-II adeno-associated viral (AAV) vector-based gene therapy clinical trials.. Produced at our plasmid DNA and viral vector CDMO centers of ...
RNA Interference based Midkine Gene Therapy for Hepatocellular Carcinoma Asian Pac J Cancer Prev. 2024 Jul 1;25(7):2371-2379. doi: 10.31557/APJCP.2024.25.7.2371. Authors Samah Mamdouh 1 ... Case-Control Studies Cell Proliferation* Female ...
In vivo versus ex vivo gene therapies for the treatment of genetic diseases and cancer.In vivo gene therapy involves direct introduction of vector (carrying the therapeutic gene) into the patient (either into or near the target organ). This strategy has achieved success in the treatment of eye diseases, neurological disorders, and hemophilia In ex vivo gene therapy, a patient's cells (e.g ...
This viewpoint discusses cost-effectiveness estimates for EtranaDez, a gene therapy for hemophilia B, using the Institute for Clinical and Economic Review's (ICER) framework for single and short-term therapies (SSTs). EtranaDez offers long-term benefits from a single administration, in contrast to the high costs and frequent dosing required by current factor IX prophylaxis. However, the ...
The study is designed to evaluate the efficacy and safety of a single infusion of the gene therapy in adults with moderately severe to severe hemophilia A. Pfizer is developing the therapy as part ...
A decade ago, Pfizer began investing heavily in gene therapy, bringing in experimental treatments for a range of genetic diseases, pumping $800 million into "state-of-the-art" manufacturing ...