An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Case Study 3: A 58-Year-Old Woman Referred for Evaluation of Suspected Alzheimer Dementia
Jeffrey maneval , m.d., jared k woods , m.d., ph.d., mel b feany , m.d., ph.d., michael b miller , m.d., ph.d., david a silbersweig , m.d., seth a gale , m.d., kirk r daffner , m.d., scott m mcginnis , m.d..
- Author information
- Copyright and License information
Send correspondence to Dr. McGinnis ( [email protected] ).
CASE PRESENTATION
A 58-year-old left-handed woman with 12 years of education was referred for further evaluation and management of progressive cognitive dysfunction. A diagnosis of Alzheimer disease (AD) was suspected by the referring neurologist on the basis of an MRI demonstrating mild temporal atrophy, EEG demonstrating bitemporal slowing, positive cerebrospinal fluid (CSF) AD biomarkers, and apolipoprotein E ( APOE ) ε4, ε4 genotype. The patient had a 4-year history of progressive cognitive symptoms. Early symptoms included difficulties recalling recent events and information, sustaining attention, and learning new tasks. She was repeatedly disoriented to the time of day; for example, she would get dressed for the day in the middle of the night. She had difficulties finding words and expressing her thoughts, with a decline in the richness of her vocabulary.
An initial clinical interview revealed that the patient had become increasingly withdrawn from family and friends. Over the year prior to evaluation at our center, she developed an array of neuropsychiatric symptoms, including depression, anxiety, and recurrent, well-formed visual hallucinations of unfamiliar people in her house. She did not feel threatened or bothered by these people and retained insight that others could not see them.
The patient had last worked as an administrative assistant approximately 3 years prior to evaluation at our center and was unable to obtain new work because of her cognitive symptoms. More recently, her husband assumed responsibility for over-seeing administration of her medications and for shopping and most meal planning and preparation. She continued to drive intermittently and to prepare breakfast independently. She remained independent in basic activities of daily living, including showering, dressing, eating, toileting, and mobility.
The patient’s mother and father lived into their 70s and 80s, respectively, and died from cancer, without any history of progressive cognitive impairment or dementia. A maternal aunt who died in her 70s had progressive cognitive impairment starting in her late 50s.
Questions: What are the diagnostic considerations based on the history? Is this presentation suggestive of AD? Would additional history be helpful?
Insidious onset and gradual progression of cognitive symptoms over the course of 4 years raises concern for a neurodegenerative disorder. In this type of scenario, it is useful to derive a three-tiered diagnostic formulation comprising neurodegenerative clinical syndrome, severity, and suspected underlying neuropathology ( 1 , 2 ). Setting aside the reports of the neuroimaging, CSF, and genetic results, aspects of the patient’s history suggesting changes in episodic memory, attention, executive function, and word retrieval could suggest a multidomain, amnesic syndrome as frequently occurs in the context of AD neuropathology. However, recurrent, well-formed visual hallucinations are very uncommon with isolated AD neuropathology and suggestive of contributions from Lewy body disease (LBD) neuropathology, as occurs in association with syndromic dementia with Lewy bodies (DLB), Parkinson disease (PD), or PD with dementia (PDD) ( 3 ). It would be useful to know whether this patient has additional history indicating other core clinical features of a DLB syndrome, including fluctuating cognition with pronounced variations in attention and alertness; REM sleep behavior disorder (RBD), suggested by dream enactment behaviors; and motor symptoms potentially reflecting parkinsonism ( 4 , 5 ). Instruments such as the Mayo Fluctuations Scale and the Queen Square Visual Hallucination Inventory provide useful questions for assessing the range of fluctuation phenomena and minor and major visual hallucinations and illusions possible in DLB ( 6 , 7 ) ( Figure 1 ). Additional supportive clinical features would include various other symptoms in the domains of motor function, sleep, neuropsychiatric function, sensory processing, and autonomic function ( 8 – 11 ).
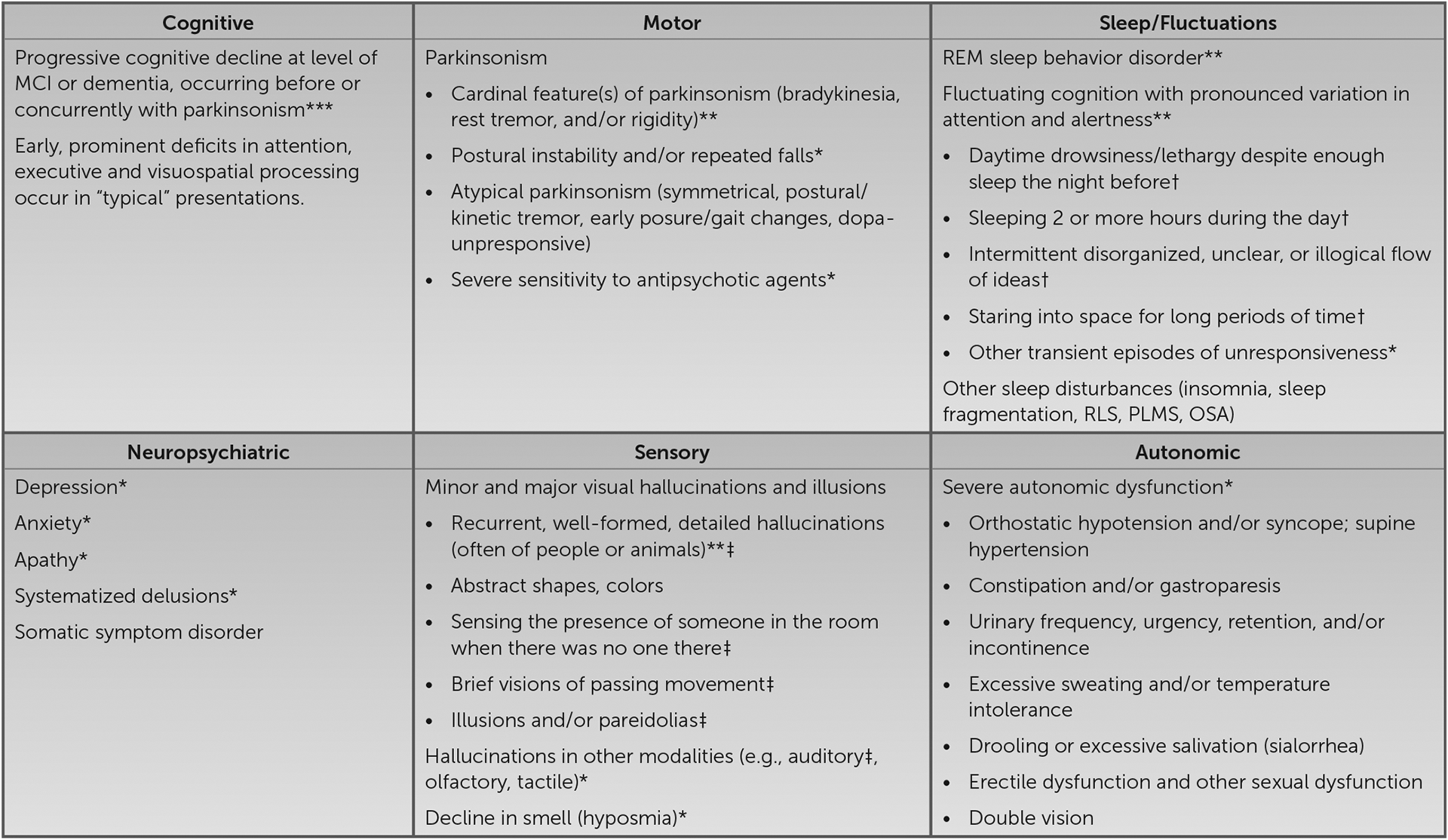
Clinical features of mild cognitive impairment and dementia with Lewy bodies a
a Asterisks denote essential (***), core (**), and supportive (*) clinical features per consensus diagnostic criteria ( 4 , 5 ). †, item from the Mayo Fluctuations Scale ( 6 ); ‡, item from the Queen Square Visual Hallucination Inventory ( 7 ); MCI, mild cognitive impairment; OSA, obstructive sleep apnea; PLMS, periodic limb movements of sleep; REM, rapid eye movement; RLS, restless legs syndrome.
Additional history revealed that the patient’s husband observed her to have periods of being in a “trance-like” state, as well as drowsiness and an increased tendency to sleep in the daytime. She was noted to have recurrent episodes of “acting out her nightmares,” at times kicking and screaming, dating back to the onset of her cognitive symptoms. Her walking had slowed, and her voice had become softer. She was slower and less coordinated when using her hands, her handwriting became smaller, and she developed intermittent tremors of her hands and arms when using them. She experienced constipation with increasing frequency and severity.
Question: What are the aims of the cognitive and neurological examinations in this context?
In the context of a history highly suggestive of DLB, one can increase diagnostic confidence by establishing a suggestive neuropsychological profile or by confirming features of parkinsonism on examination. DLB is frequently associated with early impairments in attention, executive function, and visuospatial processing ( 12 – 14 ). To assess parkinsonism, it is useful to gain familiarity with elements of the motor examination section of the Movement Disorders Society–sponsored revision of the Unified Parkinson’s Disease Rating Scale, including assessment of speech, facial expression, rigidity, finger tapping, hand movements (opening and closing), hand pronation-supination, toe tapping, leg agility (foot stomping), arising from chair, gait, posture, postural stability, global spontaneity of movement, and presence or absence of postural, kinetic, or rest tremors of the hands ( 15 ). PD syndrome is more likely to be associated with typical parkinsonism, i.e., early asymmetrical “pill-rolling” resting tremor, limb bradykinesia and rigidity that tend to be responsive to levodopa, and minimal to no early postural instability. Although typical parkinsonism can occur in DLB, atypical features occur more frequently, including the absence of resting tremor, the presence of postural-kinetic or mixed tremor, less prominent and more symmetrical early bradykinesia and rigidity, more prominent early postural instability, and reduced responsiveness to levodopa ( 16 – 18 ). Despite these distinctions, it is noteworthy that all symptomatic features of DLB can occur in PDD and vice versa, with the arbitrary distinguishing feature between the syndromes being whether cognitive dysfunction develops prior to or concurrently with parkinsonism (as in DLB and as was observed in this patient) or following parkinsonism (as in PDD) ( 4 ). Considering PD, PDD, and DLB as syndromes under the umbrella term “Lewy body disorders” allows for variability in presentations along a spectrum, from individuals with predominantly motor symptoms to those with predominantly cognitive symptoms ( 19 ). These disorders all share a common neuropathological substrate that is indistinguishable at the microscopic level but appears to differ in terms of topographical distribution and spreading patterns ( 20 ).
This patient’s mental status examination revealed grossly apparent psychomotor slowing. She scored a 24/30 on the Mini-Mental State Examination, missing 2 points on orientation to year and day of the week, 2 points on the three-item delayed word recall test (obtaining both with a category cue), and 1 point for poor pentagon copy ( 21 ). She was given a subset of the Hooper Visual Organization Test, on which she correctly identified seven of 13 objects represented in line drawings as puzzle pieces (suggesting a moderate level of visuospatial impairment) ( 22 ).
On elemental neurological examination, the patient had hypomimia, saccadic intrusions during smooth-pursuit eye movements, and slow, hypophonic speech. Strength was full in the proximal and distal muscles of the arms and legs. There was a postural-kinetic tremor of both hands, mild left-greater-than-right bradykinesia apparent on finger tapping and hand movements, and mild left-greater-than-right cogwheel rigidity in the arms. She rose from a chair easily, without the use of her arms. Her gait was mildly slow, with a narrow base and left-greater-than-right reduced arm swing. There was no retropulsion on pull testing.
Taken together, these examination results provided additional support for a DLB syndrome. The cognitive examination, though limited and nonspecific, provided some evidence of slow processing speed, impaired memory (at least at the level of retrieval), and visuospatial dysfunction. The motor examination provided unequivocal evidence of parkinsonism, some features that were typical, such as asymmetrical limb bradykinesia and cogwheel rigidity, and other features that were atypical, such as postural-kinetic tremor of the hands.
Question: What initial tests and studies are indicated?
Structural neuroimaging of the brain, preferably with MRI, is a recommended component in the initial evaluation of suspected dementia ( 23 , 24 ). When specific neurodegenerative causes of dementia are under consideration, imaging serves at least two purposes. First, it helps to assess for evidence of alternative (nondegenerative) conditions that might account for or contribute to symptoms. Second, it helps to assess for atrophy in a topographical distribution suggestive of a neurodegenerative syndrome and/or neuropathology, which can be useful in cases with possible underlying AD or frontotemporal lobar degeneration (FTLD) neuropathological changes ( 25 ). In a case such as this one, with features suggesting a DLB syndrome, imaging can be useful to assess for alternative causes of parkinsonism, such as vascular disease. While relative preservation of medial temporal lobe (MTL) regions on structural neuroimaging represents a supportive biomarker for DLB, evidence of MTL atrophy does not preclude a diagnosis of DLB, particularly considering that a DLB clinical syndrome may be associated with mixed LBD and AD neuropathological changes ( 4 , 26 ).
In cases of suspected DLB with profound fluctuations in attention, focal dyscognitive seizures are included in the differential diagnosis. Here, an EEG can be useful to distinguish between epileptiform activity (which is consistent with seizures) and prominent posterior slow-wave activity with periodic fluctuations in the pre-alpha/theta range (which is another supportive biomarker for DLB) ( 4 ).
Otherwise, a standard laboratory evaluation including comprehensive metabolic profile (CMP), vitamin B12 level, TSH, and complete blood counts (CBC) would add value in screening for potential contributing factors.
This patient’s MRI, completed approximately 9 months prior to referral, demonstrated a mild degree of T2 hyper-intense signal changes in the periventricular, subcortical and juxtacortical white matter. There was no diffusion restriction, evidence of atrophy, or abnormal enhancement ( Figure 2 ). A routine EEG demonstrated “mild intermittent bitemporal irregular slowing with no focal or generalized epileptiform features.” There were no pertinent lab result abnormalities.
Question: Are additional tests and studies indicated?
Given the presence of all four core clinical features, multiple supportive clinical features (constipation, anxiety, and depression), and a supportive biomarker (relative preservation of MTL structures on MRI) for DLB in this case, there was no strong rationale to obtain additional data to confirm the diagnosis. In cases in which a diagnosis of DLB is less clear, consideration may be given to obtaining what have been designated indicative biomarkers, including dopamine transporter SPECT or PET imaging for evidence of reduced uptake in the basal ganglia, 123 iodine-metaiodobenzylguanidine (MIBG) myocardial scintigraphy for evidence of low uptake, or polysomnography for confirmation of REM sleep without atonia ( 4 ).
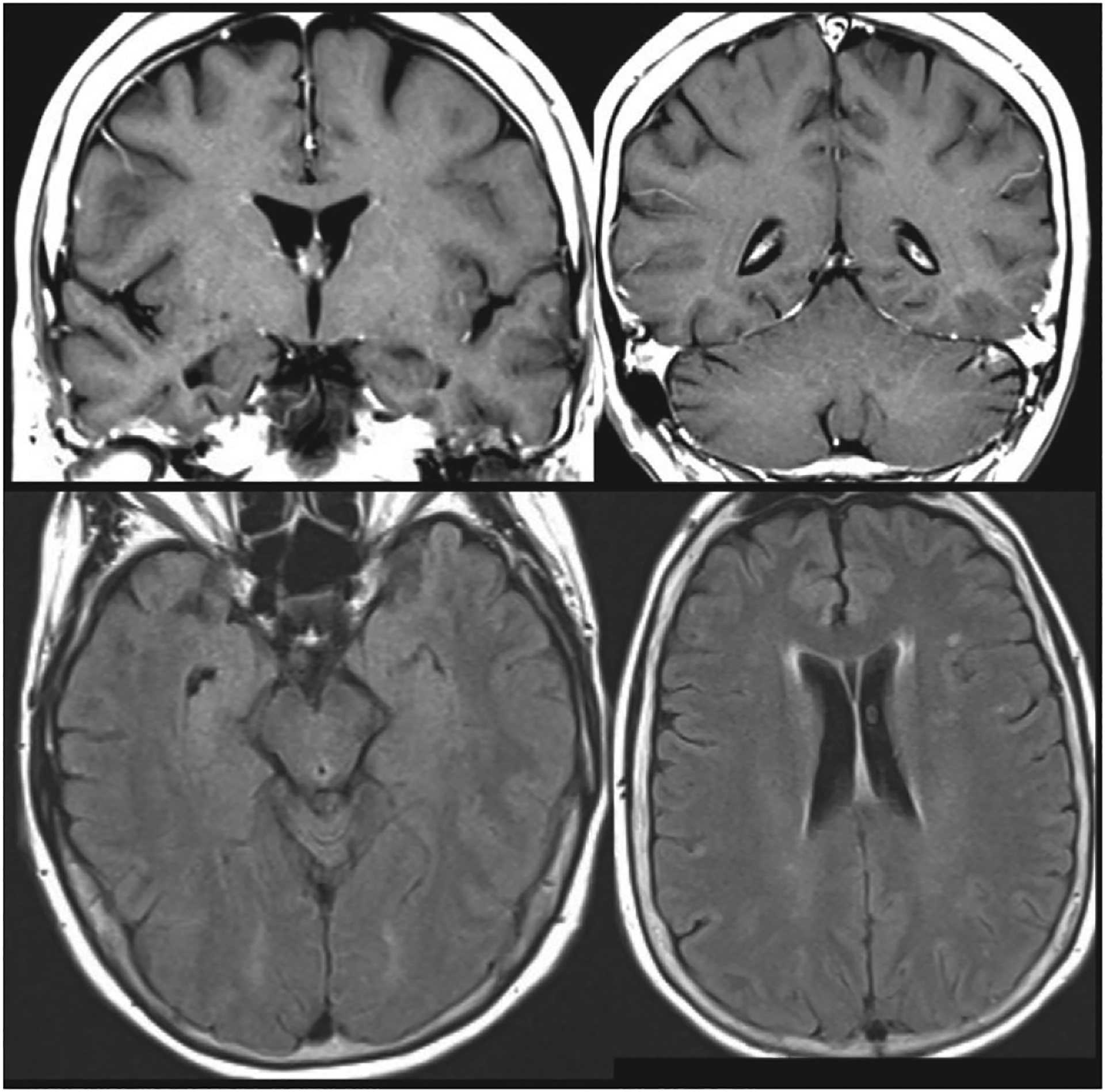
MRI of the patient’s brain obtained 9 months prior to referral and approximately 3 years after the onset of symptoms
At the time of the patient’s initial presentation, when Lewy body features were less apparent, the referring neurologist had a higher level of suspicion for an amnesic syndrome with underlying AD neuropathology than for a DLB syndrome with underlying LBD or mixed LBD-AD neuropathology. Concern for AD in a patient with mild cognitive impairment or dementia at a young age at onset (<65 years) represents an appropriate indication for obtaining CSF beta-amyloid-42 (Aβ42), total tau, and phosphorylated tau levels, as occurred in this case ( 27 ). Molecular biomarkers of AD neuropathology can also provide value in etiologically mixed presentations in which AD might contribute to an illness in which other factors are also suspected, e.g., LBD, vascular neuropathology, or both ( 28 ). Indeed, CSF evidence of underlying AD in patients with DLB has been associated with more rapid cognitive decline ( 29 ).
CSF results were suggestive of AD, because Aβ42 was low at 437.2 pg/ml, total tau was elevated at 639.7 pg/ml, the Aβ42-to-total tau index was low at 0.44, and phosphorylated tau was elevated at 83 pg/ml.
Question: Is genetic testing indicated?
Experts do not recommend routine clinical genetic testing for patients with DLB or patients with AD who lack a family history suggesting autosomal-dominant inheritance ( 4 , 30 ). In cases in which a familial autosomal-dominant neurodegenerative disorder is suggested by the clinical presentation and family history, consideration may be given for referral to a genetics counselor and potential testing for mutations in selected genes. Families with an autosomal-dominant disorder typically contain at least three affected individuals in two or more generations, with two of the individuals being first-degree relatives of the third individual, and the disorder usually involves an early age at onset. Although the presence of the APOE ε4 allele is a risk factor for both AD and DLB, its presence is neither sensitive to nor specific for either condition, and APOE genotyping is not recommended as part of a diagnostic evaluation ( 30 , 31 ). Although the utility of APOE genotyping for purposes other than diagnosis, such as clinical prognostication, has not been studied as extensively, the presence of an APOE ε4 allele in patients with DLB has been associated with greater severity of LBD neuropathology, independent of AD neuropathology, and shorter mean time between onset of cognitive symptoms and death ( 32 , 33 ).
Prior to referral, this patient tested negative for mutations in PSEN1 , PSEN2 , and APP . She was found to be a homozygous carrier of the APOE ε4 allele.
Question: What would be an appropriate diagnostic formulation?
As reviewed above, ample evidence supported a syndromic diagnosis of DLB. The patient’s loss of independence in selected instrumental activities of daily living supported staging at a level of mild dementia. Regarding suspected neuropathology, several factors supported a prediction of mixed LBD and AD neuropathology. A clinical diagnosis of DLB has greater than 95% specificity for pathological confirmation of diffuse neocortical Lewy bodies ( 34 ). Clinical and neuropathological correlation studies likewise suggest that 60%–70% of cases with a clinical diagnosis of DLB have intermediate- or high-level AD copathology ( 26 ).
Question: What are reasonable therapeutic considerations?
Because many different types of symptoms can arise in the context of DLB, it is helpful to take a systematic approach to therapeutic planning by reviewing the nature and severity of disease impact on cognition, neuropsychiatric health (e.g., mood, anxiety, and psychosis), sleep, motor function, and autonomic function. Considering the impact of symptoms within each of these domains on the patient’s quality of life and ability to carry out intended activities helps to identify and prioritize targets for pharmacological intervention ( 35 – 50 ) ( Table 1 ). Additional research is needed to establish stronger evidence for many symptomatic treatments ( 35 , 45 , 51 ). Importantly, nontrivial symptomatic benefits can frequently be obtained from discontinuing and avoiding non-essential medications with anticholinergic or dopamine receptor–blocking properties. Neuroleptic medications in particular should be avoided, given their propensity to precipitate severe, potentially life-threatening reactions ( 52 ).
Symptomatic pharmacotherapeutic considerations in dementia with Lewy bodies (DLB) a
Abbreviations: AD, Alzheimer disease; EPS, extrapyramidal symptoms; FDA, U.S. Food and Drug Administration; FTD, frontotemporal dementia; GI, gastro-intestinal; PD, Parkinson disease; PDD, Parkinson disease with dementia; QD, once daily; RBD, REM sleep behavior disorder; RLS, restless legs syndrome; SIADH, syndrome of inappropriate antidiuretic hormone secretion; VaD, vascular dementia.
Risk of GI side effects with transdermal rivastigmine is generally regarded as lower than that with oral rivastigmine.
Studies support modest improvements in global efficacy based on Clinician Global Impression of Change scale scores, without improvement in cognition and variable benefit for neuropsychiatric symptoms.
At the time of referral, the symptoms with the greatest impact on this patient’s level of function and quality of life were those involving her cognitive function, mood, anxiety, and sleep. Although meaningful in terms of diagnosis, the patient’s formed visual hallucinations and motor symptoms were not causing significant distress at presentation and therefore did not warrant pharmacological treatment. Evidence from randomized controlled trials and meta-analyses supports the efficacy of cholinesterase inhibitors for cognitive and potentially for neuropsychiatric symptoms (including anxiety, delusions, and hallucinations) in DLB patients ( 53 , 54 ). Rivastigmine and donepezil have been studied more extensively than galantamine, and the results with rivastigmine and donepezil have been comparable ( 35 , 36 ). Either would be a reasonable choice for this patient, with monitoring for sleep-related and gastrointestinal side effects ( Table 1 ).
No systematic studies of antidepressants or anxiolytics have been conducted for treatment of depression or anxiety among patients with DLB ( 55 ). Selected selective serotonin reuptake inhibitors and serotonin-norepinephrine reuptake inhibitors, such as sertraline, escitalopram, and venlafaxine, may provide benefit, although they should be used carefully (i.e., “start low and go slow”) given their potential to cause or exacerbate gastrointestinal and sleep-related problems in this population. Patients with treatment-refractory depression may benefit from repetitive transcranial magnetic stimulation or electroconvulsive therapy ( 56 , 57 ).
In cases of RBD involving frequent, disruptive, or injurious behaviors, melatonin is often well tolerated and effective in reducing dream enactment behaviors ( 58 ). Standard practice is to start at 3 mg or 5 mg and to titrate weekly in increments of 3 mg or 5 mg as needed, up to 15 mg to 18 mg nightly ( 59 ). Clonazepam may be used as a second-line treatment in severe cases; however, the potential to exacerbate cognitive dysfunction and obstructive sleep apnea should be noted.
This patient was started on transdermal rivastigmine, titrated from 4.6 mg daily to 9.5 mg daily after 1 month, and there were notable reductions in her visual hallucinations and fluctuations in attention and alertness. She did not tolerate a further increase in dose to 13.3 mg daily on account of insomnia. She derived a moderate benefit from melatonin 6 mg nightly, with respect to reducing dream enactment behaviors. Sertraline, started approximately 6 months later, was effective in reducing her anxiety at a dose of 50 mg daily. Insomnia, initially nonresponsive to medications, including trazodone and mirtazapine, improved later with an increase in the dose of melatonin to 15 mg nightly.
The patient’s clinical status deteriorated steadily despite these early symptomatic improvements. Two years after her initial behavioral neurology evaluation and 6 years after onset of symptoms, she required full-time supervision and assistance to pick out clothes and dress, and she appeared frequently frustrated and agitated. Her husband derived benefit from visits with a social worker in the behavioral neurology and neuropsychiatry unit, who provided disease-specific counseling and education, assistance with completion of medical and financial legal documents, and recommendations on care arrangements and reviewed behavioral and environment-based strategies for managing neuropsychiatric symptoms. In-home services and support from additional family members allowed her husband to continue to work on a part-time basis.
In subsequent months, the patient’s course was complicated by intermittent problems, such as dehydration, constipation, and emotional lability, and by chronic problems, including the loss of communicative abilities and mobility. She was transitioned to a memory care unit and later to skilled nursing. She died at age 63, 5 years after the initial behavioral neurology presentation and 9 years after the onset of symptoms.
NEUROPATHOLOGY
The weight of the patient’s brain was 1,160 g, which is at the lower end of the normal range for an adult female. On gross examination of the right cerebral hemisphere (the left hemisphere was banked for future research), there was mild atrophy of the anterior temporal lobe and superior temporal gyrus and no significant atrophy of the frontal, parietal, and occipital lobes. Cut sections of the midbrain revealed pallor of the substantia nigra. Similar gross depigmentation was noted in the region of the locus coeruleus in the pons.
Histologically, microscopy with hematoxylin and eosin staining confirmed a loss of pigmented neurons in the substantia nigra, as well as abundant extraneuronal pigment. There were frequent small, round eosinophilic inclusions in the neurons of the substantia nigra, representing Lewy bodies ( Figure 3 ). These were also identified with synuclein immunohistochemistry (IHC) as higher-intensity brown areas, with horseradish peroxidase chromagen–reflecting, synuclein-positive intraneuronal inclusions, which are distinct from the smaller punctate pigmented endogenous neuromelanin granules. Numerous synuclein-positive neurites were also apparent. Moderate or frequent Lewy bodies were likewise seen in the medulla, locus coeruleus, nucleus basalis of Meynert, amygdala, hippocampus, and anterior cingulate cortex.
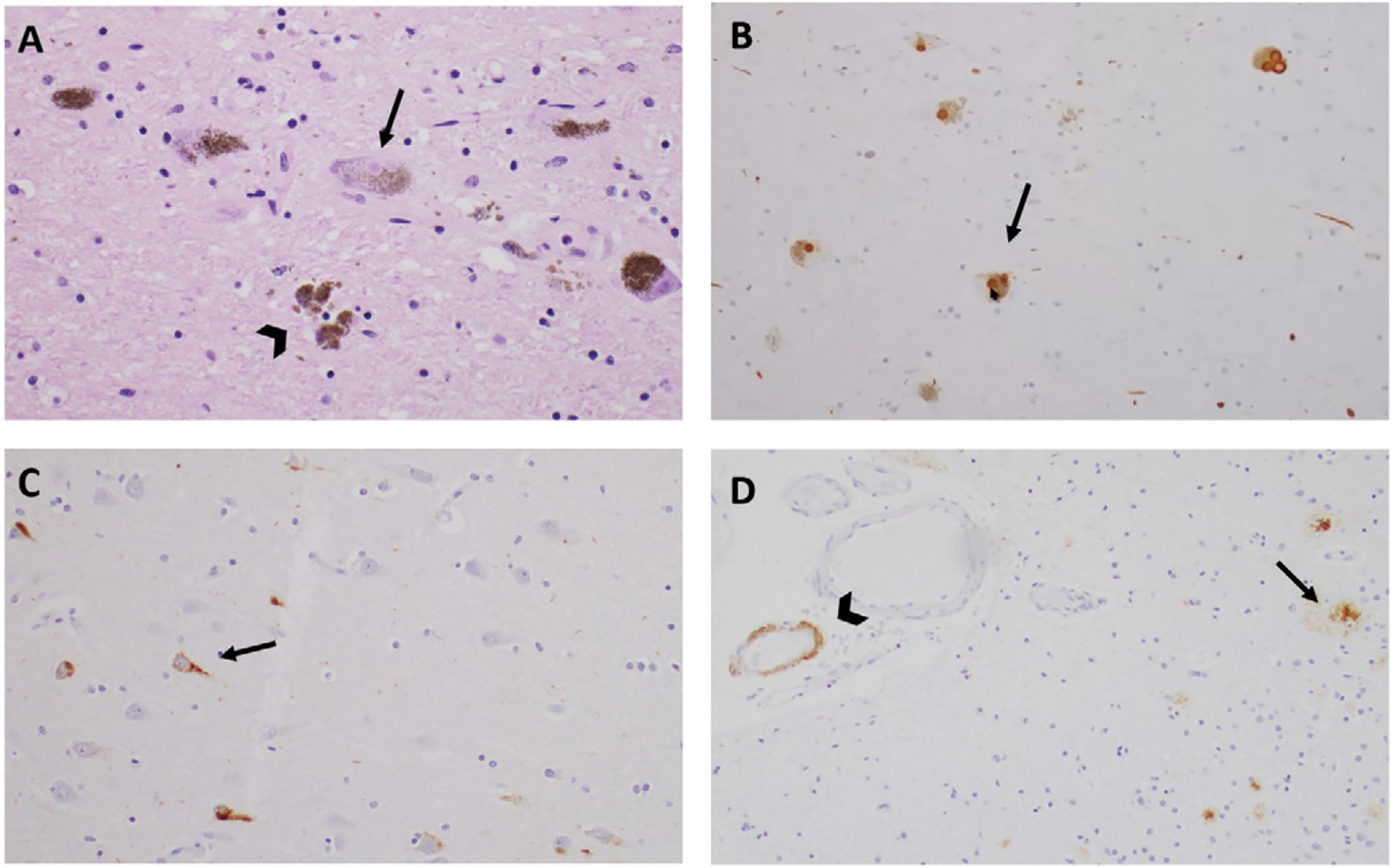
Histopathological findings for a patient with mixed Lewy body dementia and Alzheimer disease neuropathology a
a Microscopic views of the midbrain substantia nigra with hematoxylin and eosin (panel A) and synuclein immunohistochemistry (panel B) demonstrate Lewy bodies (arrows) and extraneuronal pigment (arrow-head). Tau immunohistochemistry on Ammon’s horn of the hippocampus indicates the presence of neurofibrillary tangles (panel C). β-amyloid immunohistochemistry of the frontal cortex indicates the presence of β-amyloid plaques (arrow) and cerebral amyloid angiopathy (arrowhead) (panel D).
LBD can be classified into one of three neuropathological types—brainstem predominant, limbic (transitional), or diffuse neocortical—by semiquantitative grading of Lewy body severity in selected regions in the brainstem, basal forebrain and limbic systems, and neocortex ( 60 ). In this case, the presence of moderate numbers of synuclein-positive inclusions in the frontal, temporal, and parietal cortices reflected relatively severe disease and merited the designation of diffuse neocortical LBD.
In the hippocampus, mild loss of pyramidal neurons was noted in the CA1 and CA2 regions. Higher-power microscopic examination of neurons demonstrated vacuolated cytoplasm with small eosinophilic granules, a finding that is termed granulovacuolar neurodegeneration. β-amyloid IHC revealed moderate to frequent plaques in the amygdala and hippocampus, as well as in the anterior cingulate, frontal, temporal, parietal, and occipital cortices. Tau IHC revealed frequent tau-positive neurofibrillary tangles (NFTs) and neurites within the hippocampus and amygdala; less frequent NFTs in the anterior cingulate, frontal, temporal, and parietal cortices; and rare NFTs in the occipital cortex. Taken together, these findings were consistent with stage IV neurofibrillary pathology according to the Braak and Braak ( 61 ) scheme and an intermediate level of AD neuropathological change per the National Institute on Aging–Alzheimer’s Association guidelines for the neuropathological assessment of AD ( 62 ).
β-amyloid deposition in the walls of scattered cerebral blood vessels in the hippocampus and temporal neocortex led to the additional neuropathological diagnosis of cerebral amyloid angiopathy of mild to moderate severity.
This case highlights the complexity of DLB as a multisystem disorder affecting cognition, neuropsychiatric function, sleep, motor function, and autonomic function. Symptoms in these different domains are variably present at varying levels of severity across individuals and at different time points along the disease course, which may lead to confusion in distinguishing DLB from PD in some cases (i.e., in patients who have prominent early parkinsonism) and from amnesic mild cognitive impairment or dementia due to suspected AD in others (i.e., in patients who do not have early parkinsonism). Recognizing prominent early impairments in attention and in executive and visuospatial function and proactively reviewing for fluctuations in arousal and attention, formed and minor visual hallucinations, dream enactment, and autonomic dysfunction promotes early consideration of DLB in cases without salient parkinsonism at presentation. It is useful for cognitive neurologists and neuropsychiatrists to develop a time-efficient examination of motor systems for elements of parkinsonism, to identify and characterize typical versus atypical features, and to grade severity. A systematic approach to reviewing symptom severity and the influence of symptoms on daily life across the domains potentially affected by DLB can help the clinician identify and prioritize potential targets for therapeutic intervention. Neuropsychiatric symptoms are very common, independent of motor symptoms, and tied to alterations in monoaminergic neurotransmitters ( 63 ). Published case series have suggested high rates of delusions (>75%), anxiety (65%–70%), depression (60%–65%), and apathy (55%–60%), with some symptoms tending to worsen with disease progression (delusions, hallucinations, and anxiety) and others tending to remain stable (depression) ( 8 , 52 ).
Neuropathologically, this case highlights the principle that the presence of multiple underlying neuropathologies is more the rule than the exception and that pathologies can have additive effects contributing to the severity and rate of progression of cognitive dysfunction ( 64 ). Patients with mixed LBD-AD neuropathology are more likely to have parkinsonism, visual hallucinations, RBD, and cognitive fluctuations, as well as faster rates of cognitive and functional decline than those with pure AD neuropathology ( 65 – 67 ). Patients with mixed LBD-AD neuropathology are more likely to have memory loss and less likely to have autonomic dysfunction than those with pure LBD ( 65 ). Important topics for ongoing research include understanding what factors contribute to the high coprevalence of AD and diffuse neocortical LBD pathology and understanding more fully how APOE ε4 might influence both.

Acknowledgments
This work was supported in part by NIH grants K08 AG065502 and T32 HL007627 (to Dr. Miller).
The authors report no financial relationships with commercial interests.
The authors have confirmed that details of the case have been disguised to protect patient privacy.
- 1. Dickerson BC, McGinnis SM, Xia C, et al. : Approach to atypical Alzheimer’s disease and case studies of the major subtypes. CNS Spectr 2017; 22:439–449 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 2. McGinnis SM, Stern AM, Woods JK, et al. : Case study 1: a 55-year-old woman with progressive cognitive, perceptual, and motor impairments. J Neuropsychiatry Clin Neurosci 2022; 34:8–15 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Ala TA, Yang KH, Sung JH, et al. : Hallucinations and signs of parkinsonism help distinguish patients with dementia and cortical Lewy bodies from patients with Alzheimer’s disease at presentation: a clinicopathological study. J Neurol Neurosurg Psychiatry 1997; 62:16–21 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 4. McKeith IG, Boeve BF, Dickson DW, et al. : Diagnosis and management of dementia with Lewy bodies: fourth consensus report of the DLB Consortium. Neurology 2017; 89:88–100 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 5. McKeith IG, Ferman TJ, Thomas AJ, et al. : Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology 2020; 94:743–755 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 6. Ferman TJ, Smith GE, Boeve BF, et al. : DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology 2004; 62:181–187 [ DOI ] [ PubMed ] [ Google Scholar ]
- 7. Williams DR, Warren JD, Lees AJ: Using the presence of visual hallucinations to differentiate Parkinson’s disease from atypical parkinsonism. J Neurol Neurosurg Psychiatry 2008; 79:652–655 [ DOI ] [ PubMed ] [ Google Scholar ]
- 8. Borroni B, Agosti C, Padovani A: Behavioral and psychological symptoms in dementia with Lewy-bodies (DLB): frequency and relationship with disease severity and motor impairment. Arch Gerontol Geriatr 2008; 46:101–106 [ DOI ] [ PubMed ] [ Google Scholar ]
- 9. Mendoza-Velasquez JJ, Flores-Vazquez JF, Barron-Velazquez E, et al. : Autonomic dysfunction in alpha-synucleinopathies. Front Neurol 2019; 10:363. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 10. Onofrj M, Bonanni L, Manzoli L, et al. : Cohort study on somatoform disorders in Parkinson disease and dementia with Lewy bodies. Neurology 2010; 74:1598–1606 [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Terzaghi M, Arnaldi D, Rizzetti MC, et al. : Analysis of video-polysomnographic sleep findings in dementia with Lewy bodies. Mov Disord 2013; 28:1416–1423 [ DOI ] [ PubMed ] [ Google Scholar ]
- 12. Calderon J, Perry RJ, Erzinclioglu SW, et al. : Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2001; 70:157–164 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Mori E, Shimomura T, Fujimori M, et al. : Visuoperceptual impairment in dementia with Lewy bodies. Arch Neurol 2000; 57:489–493 [ DOI ] [ PubMed ] [ Google Scholar ]
- 14. Salmon DP, Galasko D, Hansen LA, et al. : Neuropsychological deficits associated with diffuse Lewy body disease. Brain Cogn 1996; 31:148–165 [ DOI ] [ PubMed ] [ Google Scholar ]
- 15. Goetz CG, Tilley BC, Shaftman SR, et al. : Movement Disorder Society–sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008; 23:2129–2170 [ DOI ] [ PubMed ] [ Google Scholar ]
- 16. Louis ED, Klatka LA, Liu Y, et al. : Comparison of extrapyramidal features in 31 pathologically confirmed cases of diffuse Lewy body disease and 34 pathologically confirmed cases of Parkinson’s disease. Neurology 1997; 48:376–380 [ DOI ] [ PubMed ] [ Google Scholar ]
- 17. Burn DJ, Rowan EN, Allan LM, et al. : Motor subtype and cognitive decline in Parkinson’s disease, Parkinson’s disease with dementia, and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2006; 77:585–589 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 18. Onofrj M, Varanese S, Bonanni L, et al. : Cohort study of prevalence and phenomenology of tremor in dementia with Lewy bodies. J Neurol 2013; 260:1731–1742 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 19. Lippa CF, Duda JE, Grossman M, et al. : DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology 2007; 68:812–819 [ DOI ] [ PubMed ] [ Google Scholar ]
- 20. Jellinger KA: Dementia with Lewy bodies and Parkinson’s disease-dementia: current concepts and controversies. J Neural Transm 2018; 125:615–650 [ DOI ] [ PubMed ] [ Google Scholar ]
- 21. Folstein MF, Folstein SE, McHugh PR: “Mini-Mental State”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12:189–198 [ DOI ] [ PubMed ] [ Google Scholar ]
- 22. Hooper HE: Hooper Visual Organization Test Manual. Los Angeles, Western Psychological Services, 1983 [ Google Scholar ]
- 23. Knopman DS, DeKosky ST, Cummings JL, et al. : Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1143–1153 [ DOI ] [ PubMed ] [ Google Scholar ]
- 24. McKhann GM, Knopman DS, Chertkow H, et al. : The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7:263–269 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. McGinnis SM: Neuroimaging in neurodegenerative dementias. Semin Neurol 2012; 32:347–360 [ DOI ] [ PubMed ] [ Google Scholar ]
- 26. Irwin DJ, Grossman M, Weintraub D, et al. : Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet Neurol 2017; 16:55–65 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 27. Shaw LM, Arias J, Blennow K, et al. : Appropriate use criteria for lumbar puncture and cerebrospinal fluid testing in the diagnosis of Alzheimer’s disease. Alzheimers Dement 2018; 14:1505–1521 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 28. Johnson KA, Minoshima S, Bohnen NI, et al. : Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. Alzheimers Dement 2013; 9: e1–16 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 29. Abdelnour C, van Steenoven I, Londos E, et al. : Alzheimer’s disease cerebrospinal fluid biomarkers predict cognitive decline in Lewy body dementia. Mov Disord 2016; 31:1203–1208 [ DOI ] [ PubMed ] [ Google Scholar ]
- 30. Goldman JS, Hahn SE, Catania JW, et al. : Genetic counseling and testing for Alzheimer disease: joint practice guidelines of the American College of Medical Genetics and the National Society of Genetic Counselors. Genet Med 2011; 13:597–605 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 31. Vergouw LJM, van Steenoven I, van de Berg WDJ, et al. : An update on the genetics of dementia with Lewy bodies. Parkinsonism Relat Disord 2017; 43:1–8 [ DOI ] [ PubMed ] [ Google Scholar ]
- 32. Dickson DW, Heckman MG, Murray ME, et al. : APOE ε4 is associated with severity of Lewy body pathology independent of Alzheimer pathology. Neurology 2018; 91:e1182–e1195 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Graff-Radford J, Lesnick TG, Boeve BF, et al. : Predicting survival in dementia with Lewy bodies with hippocampal volumetry. Mov Disord 2016; 31:989–994 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 34. Nelson PT, Jicha GA, Kryscio RJ, et al. : Low sensitivity in clinical diagnoses of dementia with Lewy bodies. J Neurol 2010; 257: 359–366 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Stinton C, McKeith I, Taylor JP, et al. : Pharmacological management of Lewy body dementia: a systematic review and meta-analysis. Am J Psychiatry 2015; 172:731–742 [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Wang HF, Yu JT, Tang SW, et al. : Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies: systematic review with meta-analysis and trial sequential analysis. J Neurol Neurosurg Psychiatry 2015; 86: 135–143 [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Pakrasi S, Thomas A, Mosimann UP, et al. : Cholinesterase inhibitors in advanced Dementia with Lewy bodies: increase or stop? Int J Geriatr Psychiatry 2006; 21:719–721 [ DOI ] [ PubMed ] [ Google Scholar ]
- 38. Ridha BH, Josephs KA, Rossor MN: Delusions and hallucinations in dementia with Lewy bodies: worsening with memantine. Neurology 2005; 65:481–482 [ DOI ] [ PubMed ] [ Google Scholar ]
- 39. Sabbagh MN, Hake AM, Ahmed S, et al. : The use of memantine in dementia with Lewy bodies. J Alzheimers Dis 2005; 7:285–289 [ DOI ] [ PubMed ] [ Google Scholar ]
- 40. Culo S, Mulsant BH, Rosen J, et al. : Treating neuropsychiatric symptoms in dementia with Lewy bodies: a randomized controlled-trial. Alzheimer Dis Assoc Disord 2010; 24:360–364 [ DOI ] [ PubMed ] [ Google Scholar ]
- 41. Tariot PN, Cummings JL, Soto-Martin ME, et al. : Trial of pimavanserin in dementia-related psychosis. N Engl J Med 2021; 385: 309–319 [ DOI ] [ PubMed ] [ Google Scholar ]
- 42. Murata M, Odawara T, Hasegawa K, et al. : Adjunct zonisamide to levodopa for DLB parkinsonism: a randomized double-blind phase 2 study. Neurology 2018; 90:e664–e672 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Zhang W, Chen XY, Su SW, et al. : Exogenous melatonin for sleep disorders in neurodegenerative diseases: a meta-analysis of randomized clinical trials. Neurol Sci 2016; 37:57–65 [ DOI ] [ PubMed ] [ Google Scholar ]
- 44. Lapid MI, Kuntz KM, Mason SS, et al. : Efficacy, safety, and tolerability of armodafinil therapy for hypersomnia associated with dementia with Lewy bodies: a pilot study. Dement Geriatr Cogn Disord 2017; 43:269–280 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Taylor JP, McKeith IG, Burn DJ, et al. : New evidence on the management of Lewy body dementia. Lancet Neurol 2020; 19: 157–169 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 46. Jost WH, Friedman A, Michel O, et al. : SIAXI: placebo-controlled, randomized, double-blind study of incobotulinumtoxinA for sialorrhea. Neurology 2019; 92:e1982–e1991 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 47. Arbouw MEL, Movig KLL, Koopmann M, et al. : Glycopyrrolate for sialorrhea in Parkinson disease: a randomized, double-blind, crossover trial. Neurology 2010; 74:1203–1207 [ DOI ] [ PubMed ] [ Google Scholar ]
- 48. Fasano A, Visanji NP, Liu LWC, et al. : Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol 2015; 14:625–639 [ DOI ] [ PubMed ] [ Google Scholar ]
- 49. Peyronnet B, Vurture G, Palma JA, et al. : Mirabegron in patients with Parkinson disease and overactive bladder symptoms: a retrospective cohort. Parkinsonism Relat Disord 2018; 57:22–26 [ DOI ] [ PubMed ] [ Google Scholar ]
- 50. Palma JA, Kaufmann H: Treatment of autonomic dysfunction in Parkinson disease and other synucleinopathies. Mov Disord 2018; 33:372–390 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Boot BP, McDade EM, McGinnis SM, et al. : Treatment of dementia with Lewy bodies. Curr Treat Options Neurol 2013; 15:738–764 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. McKeith I, Fairbairn A, Perry R, et al. : Neuroleptic sensitivity in patients with senile dementia of Lewy body type. BMJ 1992; 305: 673–678 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. McKeith I, Del Ser T, Spano P, et al. : Efficacy of rivastigmine in dementia with Lewy bodies: a randomised, double-blind, placebo-controlled international study. Lancet 2000; 356:2031–2036 [ DOI ] [ PubMed ] [ Google Scholar ]
- 54. Mori E, Ikeda M, Kosaka K, et al. : Donepezil for dementia with Lewy bodies: a randomized, placebo-controlled trial. Ann Neurol 2012; 72:41–52 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Ballard C, Aarsland D, Francis P, et al. : Neuropsychiatric symptoms in patients with dementias associated with cortical Lewy bodies: pathophysiology, clinical features, and pharmacological management. Drugs Aging 2013; 30:603–611 [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Takahashi S, Mizukami K, Yasuno F, et al. : Depression associated with dementia with Lewy bodies (DLB) and the effect of somatotherapy. Psychogeriatrics 2009; 9:56–61 [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Rasmussen KG Jr., Russell JC, Kung S, et al. : Electroconvulsive therapy for patients with major depression and probable Lewy body dementia. J ECT 2003; 19:103–109 [ DOI ] [ PubMed ] [ Google Scholar ]
- 58. Boeve BF, Silber MH, Ferman TJ: Melatonin for treatment of REM sleep behavior disorder in neurologic disorders: results in 14 patients. Sleep Med 2003; 4:281–284 [ DOI ] [ PubMed ] [ Google Scholar ]
- 59. McGrane IR, Leung JG, St Louis EK, et al. : Melatonin therapy for REM sleep behavior disorder: a critical review of evidence. Sleep Med 2015; 16:19–26 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. McKeith IG, Dickson DW, Lowe J, et al. : Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 2005; 65:1863–1872 [ DOI ] [ PubMed ] [ Google Scholar ]
- 61. Braak H, Braak E: Staging of Alzheimer’s disease–related neurofibrillary changes. Neurobiol Aging 1995; 16:271–278 [ DOI ] [ PubMed ] [ Google Scholar ]
- 62. Hyman BT, Phelps CH, Beach TG, et al. : National Institute on Aging–Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimers Dement 2012; 8: 1–13 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 63. Vermeiren Y, Van Dam D, Aerts T, et al. : The monoaminergic footprint of depression and psychosis in dementia with Lewy bodies compared to Alzheimer’s disease. Alzheimers Res Ther 2015; 7:7. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Bennett DA, Buchman AS, Boyle PA, et al. : Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis 2018; 64: S161–S189 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 65. Chatterjee A, Hirsch-Reinshagen V, Moussavi SA, et al. : Clinicopathological comparison of patients with autopsy-confirmed Alzheimer’s disease, dementia with Lewy bodies, and mixed pathology. Alzheimers Dement 2021; 13:e12189. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Gu Y, Kociolek A, Fernandez KK, et al. : Clinical trajectories at the end of life in autopsy-confirmed dementia patients with Alzheimer disease and Lewy bodies pathologies. Neurology 2022; 98: e2140–e2149 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Malek-Ahmadi M, Beach TG, Zamrini E, et al. : Faster cognitive decline in dementia due to Alzheimer disease with clinically undiagnosed Lewy body disease. PLoS One 2019; 14: e0217566. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- View on publisher site
- PDF (1.1 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections

IMAGES