An official website of the United States government
Official websites use .gov A .gov website belongs to an official government organization in the United States.
Secure .gov websites use HTTPS A lock ( Lock Locked padlock icon ) or https:// means you've safely connected to the .gov website. Share sensitive information only on official, secure websites.
- Publications
- Account settings
- Advanced Search
- Journal List


Towards effective clinical decision support systems: A systematic review
Francini hak, tiago guimarães, manuel santos.
- Author information
- Article notes
- Copyright and License information
Competing Interests: The authors have declared that no competing interests exist.
* E-mail: [email protected]
Received 2020 Dec 6; Accepted 2022 Jul 27; Collection date 2022.
This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Clinical Decision Support Systems (CDSS) are used to assist the decision-making process in the healthcare field. Developing an effective CDSS is an arduous task that can take advantage from prior assessment of the most promising theories, techniques and methods used at the present time.
To identify the features of Clinical Decision Support Systems and provide an analysis of their effectiveness. Thus, two research questions were formulated: RQ1—What are the most common trend characteristics in a CDSS? RQ2—What is the maturity level of the CDSS based on the decision-making theory proposed by Simon?
AIS e-library, Decision Support Systems journal, Nature, PlosOne and PubMed were selected as information sources to conduct this systematic literature review. Studies from 2000 to 2020 were chosen covering search terms in CDSS, selected according to defined eligibility criteria. The data were extracted and managed in a worksheet, based on the defined criteria. PRISMA statements were used to report the systematic review.
The outcomes showed that rule-based module was the most used approach regarding knowledge management and representation. The most common technological feature adopted by the CDSS were the recommendations and suggestions. 19,23% of studies adopt the type of system as a web-based application, and 51,92% are standalone CDSS. Temporal evolution was also possible to visualize. This study contributed to the development of a Maturity Staging Model, where it was possible to verify that most CDSS do not exceed level 2 of maturity.
The trend characteristics addressed in the revised CDSS were identified, compared to the four predefined groups. A maturity stage model was developed based on Simon’s decision-making theory, allowing to assess the level of maturity of the most common features of the CDSS. With the application of the model, it was noticed that the phases of choice and implementation are underrepresented. This constitutes the main gap in the development of an effective CDSS.
Introduction
In the day-to-day routine of healthcare units, the domain professionals interact with hundreds of patients. Naturally, it is of great importance that all this interaction is transformed into records, whether clinical or administrative, which in turn are transformed into data, information, and, hopefully, knowledge. Despite technological developments, these records are still kept in physical format, which creates a delay in the work performed when compared to digital versions [ 1 ].
Friedman [ 2 ] boasted a theorem in which it shows that an individual or group working with the contribution of a technological resource of information, has a better performance compared to a job without such assistance. For this functional theorem to be verified, the information resources must be valid and reliable and the user must know how to handle it properly.
The electronic representation of clinical records are embodied by Electronic Health Records (EHR), which aim, in particular, to eliminate the use of paper in the healthcare field. Notwithstanding, the representation of electronic health data tends to be more than just a substitute for paper [ 1 ]. HIMSS [ 3 ] shows that a healthcare information system must have the ability to generate a complete view of the clinical record at a meeting with a patient, including decision evidence-based support, quality management and results reporting, apart from the support of other activities related to direct or indirect care by means of a shared area. Thereby, it is evident that for a healthcare information system to be effective, it is crucial the existence of an integrated component of support in decision-making processes.
The concept of Decision Support (DS) is equivalent to the activity that assists a given user following a certain purpose. Thus, a Decision Support System (DSS) turns this activity into a system-based format in an efficient and reliable way, composing models and techniques through a knowledge representation infrastructure [ 4 ]. In this sense, a DSS portrays a system designed to support a professional in obtaining knowledge and making decisions in the specific area, therefore, diminishing uncertainties during the decision-making process [ 5 ].
In the healthcare field, the decision-making support activity is designed as Clinical Decision Support (CDS). The representation of this activity in a computerized-based format is translated into a Clinical Decision Support System (CDSS), where it provides all the information and desired knowledge that facilitate the daily tasks of healthcare providers and guarantee an improvement in the quality of services.
As a clinical knowledge-based system, the treatment of information and the process of knowledge extraction are considerable aspects for a Clinical Decision Support System to achieve its objectives. Some features are mandatory in a CDSS, but not all are known and some may be missed and contribute to system failures [ 6 ].
According to Simon, the decision-making process is the heart of any organization and it influences all processes integrated into it [ 7 ]. The decision-making process can be outlined using various models and theorems. Within the scope of this study, the Bounded Rationality theory was adopted as a reference to Herbert Simon’s work [ 8 ]. It is stated that people act in a rational way according to the knowledge and perception that they get. In an initial phase, this model approached three stages: Intelligence, Design and Choice. Later on, a new stage was added by Sprague and Carlson [ 9 ], resulting in the implementation phase. Thus, the four phases resulted by Simon’s work [ 8 ], redound to model decision making process of an Intelligent Decision Support System.
Previous systematic review studies (SRs) have addressed the use of a Clinical Decision Support System to assess its use and effectiveness [ 10 – 12 ], but applied to a specific workflow. Instead, this systematic review aims to analyse a set of articles that address a CDSS in order to identify the trends of the characteristics for its conception. In addition, we also identify other aspects such as the purpose of care and the recipient of the intervention. Furthermore, in order to enrich the outcomes obtained from the review, we trace a general trend of a CDSS, relating it with the four phases proposed by Simon.
This article is structured in four sections. First, an introduction to the topic of study is presented. Secondly, the landscape of the methods used to make the Systematic Review. The third section presents the results of the Systematic Review. The fourth section is dedicated to discussing the results obtained. At last, in section five, conclusions are drawn while leaving open doors for future work.
Decision-making theory
Decision-making is one of the main processes dealt within an organization [ 13 ]. This process can be approached using different models and theories. In the present study, the bounded rationality theory proposed by Simon will be focused.
Herbert Simon [ 8 ] was an economist who carried out research involving several areas, such as psychology, computing and management. One of Simon’s great contributions to scientific research was the theory of bounded rationality, where Simon redefines human rationality arguing that people act rationally according to the knowledge and perception they obtain. To formalize his theory, Simon established four phases that define the decision-making process, as shown in Fig 1 .
Fig 1. Iterative phases of the decision-making process.
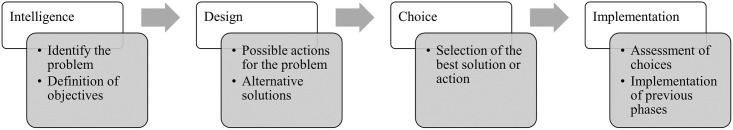
In Intelligence phase, problems are identified and the purpose of action involving the decision is determined. In the Design phase, possible solutions are designed and alternatives are created to solve the problem. Consequently, in the Choice phase, the alternative that most satisfies the purpose of action will be chosen. Years later, Turban [ 14 ] was responsible for reformulating Simon’s theory by adding a fourth phase in order to assess the implementation process. Thus, the Implementation phase results from the joining of the three previous phases put into practice. It should be noted that the decision-making is a sequential and iterative process, which requires the completion of all phases to reach the final phase and an ongoing review of the same.
Intelligence—Process of formalization of the problem and definition of decision conditions. The decisions to be made with the help of the proposed system and the benefits it will bring are still unclear and not understood. In this phase, reality is examined in order to model the existing information for decomposing the problem and determining its properties. The intelligence phase ends with a problem statement.
Design—At this stage it is necessary to have the problem defined to search for alternatives or available options, where possible courses of action are analysed and validated. Problem-situation models are built to explore alternative solutions. For this, it is necessary to have well-defined and declared selection criteria as a result for the evaluation of potential solutions that will be identified (includes techniques, technologies, format, integration, functionalities, etc). Several possible outcomes can be considered for each alternative, each with a certain probability of occurrence. In cases where decisions are made with uncertainty, there are multiple outcomes for each alternative.
Choice—In the choice phase, all alternatives are researched, evaluated, and one is defined as the recommended solution. In pursuit of goals, a course of action that is good enough is selected. Computer models or critical success factors can be used as techniques to evaluate the recommended solution. Normative models can use either the analytical model or an exhaustive and complete enumeration model. The solution is tested and once the proposed solution appears viable, a decision can be made.
Implementation—This phase is the validation of all previous phases. If the adopted solution seems good enough, it can be implemented. Successful implementation results in solving the original problem. In case of failure, it must be returned to the previous phases.
The systematic review was conducted based on Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statements [ 15 ], followed by a checklist and a flow diagram.
Information sources
Five online data sources related to research in health information systems were selected. First, a search was performed on Scimago [ 16 ] comprising two areas of study: information systems and multidisciplinary. From this, journals with Q1 quartile in the last 5 years and with an impact factor greater than 150 were analysed. Journals that had the potential to have articles more related to the purpose of this study and that were already known by the authors were selected: Decision Support Systems, Nature Research Journal and Plos One. To complement the study, two public repositories were chosen, such as: the PubMed library (medline) and the Association of Information Systems (AIS) e-library. The Table 1 shows information about the selected data source types.
Table 1. Information of selected data sources.
Source: Scimago Journal and Country Rank via www.scimagojr.com , accessed on May 13, 2022.
Eligibility criteria and search strategy
To select the studies for the development of the systematic review, eligibility criteria were defined: (i) published studies in article format; (ii) open access or free full text; (iii) written in English; (iii) articles addressing a decision support systems in healthcare; (iv) computerized or electronic decision support systems. To meet these criteria, search filters were applied. The selected articles must address practical cases of a specific clinical decision support system, therefore, articles of the literature review type or that did not address a particular CDS system were excluded.
The search strategy was performed considering an ordering from the most recent publications to the oldest ones, restricting to a range from 2000/01/01 to 2020/12/31. To identify studies of the desired scope, the query terms were: ((everything:“Clinical Decision Support System”) OR everything:“Clinical Decision Support”) OR everything:“Decision Support System in Healthcare”. The title, abstract and keywords were the primary strategy for analyzing each scientific publication. When these parameters were not sufficient, a complete reading of each article was made, in order to guarantee the eligibility of inclusion criteria.
Data extraction and management
The screening process was carried out by two authors (FH and TG) independently, by reading the titles, abstracts and keywords of the publications of the search results. When the criteria were met, the full text was read. In moments of disagreement or doubt, the third reviewer (MS) took a stand and contributed to the discussion.
Through the review of eligible articles, data extraction was performed by two reviewers (FH and TG) and focused on four characteristics of a CDSS, defined as: knowledge management and representation technique; technological resources integrated into the system; the type of system; and system integration. These four categories were considered by the authors as the most relevant for the purpose of this study. In addition, the following information was extracted: clinical setting, study design, recipient of intervention, and purpose of care. Data extraction was performed through the textual analysis of the articles and the search for keywords. For better management and analysis, the extracted data were recorded on an appropriate sheet (see S1 File ).
To answer the second research question of this study, it was identified in which phase of the decision-making theory the CDSS of the reviewed articles was found. Thus, based on the literature on decision-making theory [ 17 , 18 ] and the definitions of each phase, the authors considered a set of criteria for the assignment of each phase, as shown in Table 2 . For a system to be classified in a certain phase, it must fulfill at least one of the conditions indicated.
Table 2. Choice of criterias for the classification of Simon’s phases.
Note: The conditions presented are of disjunction, that is, at least one of them must be met for the system to be classified in a certain phase.
Quality assessment and data synthesis
All the reviewed studies portray a Clinical Decision Support System (CDSS) designed for a specific purpose. The key points for conducting this systematic review were to identify the trends of the CDSS developed regarding their type of system, integration, representation and formulation of knowledge, and their technological features.
The reporting quality was made following the PRISMA checklist. Publications considered eligible for the analysis were assessed for the methodological quality, meeting the inclusion criteria, risk of bias, assumptions and simplifications, and clarified evidence-based results. The reviewers did not apply any methodological assessment tool.
After defining the five data sources, the reviewers selected a set of publications within the scope of Clinical Decision Support Systems, through the search strategy and the inclusion criteria previously defined. The outcomes presented through tables and narrative summary characterize the different Clinical Decision Support Systems presented in the selected studies, in order to trace a global trend. The data from the matching results were pooled out and evaluated, based on the total value of studies and the probability of occurrence. The reviewers noted the presence of clinical and statistical heterogeneity among the studies.
Study selection
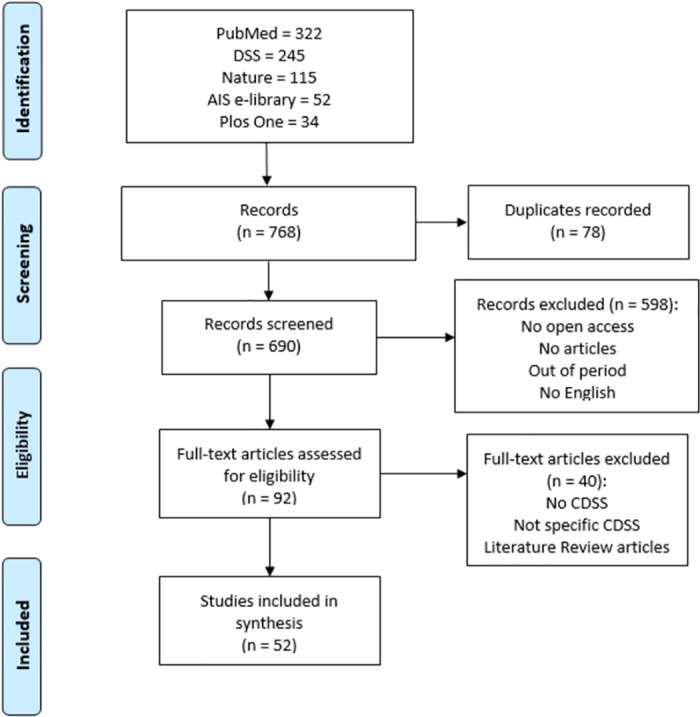
The study selection was carried out following the PRISMA statement [ 15 ], using a checklist (see S2 File ) and a flow diagram represented in Fig 2 ( S3 File ). 768 records were identified by searching five databases considered most influential for the purpose. After removing duplicate records, 690 records were screened to read the title, abstract and keywords. Lastly, the articles that did not meet the selection and search criteria were excluded, comprising: non-article format; unpublished articles; not in english; out of period; not open access. The selected articles were read in full to assess their eligibility. Finally, 52 articles met the research questions and were elected eligible and appropriate to carry out the systematic review, embracing a specific Clinical Decision Support System and not literature review articles.
Fig 2. Flow diagram of study selection for systematic review.
Study characteristics
The studies that met the inclusion criteria were characterized according to a set of attributes. The period covered between the studies was from 2000 to 2020, with 50% from 2020, giving priority to the most recent ones.
The studies were searched in the five previously selected data sources, with eighteen studies from PubMed (34,62%), eleven studies from Decision Support Systems journal (21,15%), ten studies from PlosOne (19,23%), and five studies from Nature (9,62%). We note a diversity in the countries that developed the studies, covering four different continents. However, the majority stand out to the United States of America, representing nineteen studies (34,62%).
Studies realized in any healthcare setting were the most frequent ones (28,85%), followed by hospital setting (21,15%) and hospital academic centers (21,15%). The most common recipients of the intervention are, directly, general practitioners (32,69%). Studies reveal that CDSS also has an action on patients, but mostly, indirectly.
In general, the study design of the reviews articles, demonstrates the evaluation of the effectiveness of the CDSS regarding its cost, implementation and usability. In addition, the review showed that the CDSS addressed have an associated clinical intervention, with the most common purpose of care being a specific workflow (32,69%), followed by a specific patient disease (15,09%).
The information extracted from the studies that were considered relevant for the desired purpose generated a set of outcomes, based on the characterization of a Clinical Decision Support System (CDSS).
Knowledge management . The representation and formulation of the acquired knowledge is one of the most important steps in the development of a CDSS. In order to identify the most used techniques for knowledge management, we have identified the approaches used in studies of the systematic review, as shown in Table 3 . Clinical Practice Guidelines (38,46%), rule-based module (40,39%) and algorithmic logic (38,46%), were the most used approaches to knowledge management in the designing of a CDSS. These techniques were used individually or in combination with others. The formulation of a knowledge base was also identified in thirteen studies (25%), in combination with other techniques. Inference engines were identified in seven studies (13,46%), which were also considered in some studies, as reasoning engines. Three studies (5,77%) provided if/then statements, and eight studies (15,39%) applied methods based on variables. Terminology standards and clinical classification systems were present in five studies (9,62%), applying, in particular, the ICD-10 system and the HL7 communication protocol. Bayesian and neural networks were used in two different articles (3,85% each). Finally, three studies (5,77%) used data mining techniques to reproduce the desired knowledge. Two studies [ 19 , 20 ] were excluded from pooling, as the method used in the knowledge representation process was not identified.
Table 3. Outcomes of knowledge representation and management.
Technological features . Technological interventions represent the features that contribute to the system achieving its purpose. The most common technological feature approached in the CDSS is recommendation and suggestion feature, identified in twenty four studies (46,15%), as shown in Table 4 . Information management and monitoring are the second most common feature in the systems, covering eighteen studies (46,15%). The third most desired feature is alerts, notifications and reminders, covering fourteen studies (26,92%). Therefore, it follows the purpose of reducing errors as mentioned in eleven studies (21,15%). Eight articles (15,38%) design the CDSS for assessment purposes. The prediction feature is also used for eight studies for different purposes (15,38%). The automation and prioritization of processes is considered to be of great importance and is addressed by seven articles (13,46%). The triggering of events is addressed by four studies (7,69%). The standardization of the clinical process is desired by four studies (7,69%). Three articles (5,77%) highlighted the calculation and scoring methods as features of a CDSS. Finally, the cost and time reduction is seen as a main feature in two studies (3,84%).
Table 4. Outcomes of technological features.
System integration . The results related to the integration of the Clinical Decision Support System (CDSS), showed that 51,92% of the articles (twenty-seven) use a standalone system, as shown in Table 5 . The remaining studies show that their CDSS are integrated with another system, as well as using the information from these systems. Thus, eleven studies (21,15%) reveal that they integrate their CDSS with an Electronic Health Record (EHR) system; five studies (9,62%) integrate their system with a specific health information system; four studies (7,69%) integrate with a Computerized Provider Order Entry (CPOE) system; in this sequence, three studies (5,77%) integrate their CDSS with both EHR and CPOE; two studies differentiate their integration with an Electronic Medical Record (EMR) system.
Table 5. Outcomes of system integration.
Type of system . The typology of a Clinical Decision Support System (CDSS) differs in several technological aspects, considering both the presentation of the user interface and the technique it is based on. The type of systems identified in the review of studies is represented in Table 6 . Most studies (19.23%) use a web-based application to present the system interface to the user. Nine studies (17,31%) describe their CDSS as a computerized tool, not specifying the type of software or interface used. Eight studies (15,38%) develop their decision support system in a software application. Regarding the logic used, five studies (9,62%) are based on machine learning techniques, and four studies (7,69%) are artificial intelligence-based. Three studies (5,77%) develop their CDSS in a mobile application and, still, four studies (7,69%) cover a mobile and web application. Two studies (3,85%) describe their CDSS as knowledge-based, and other two studies (3,85%) present the CDSS as data analytics. Other types of systems were considered in unique studies, such as cloud computing (1,92%), data-layer infraestructure (1,92%), image retrieval expert system (1,92%), user interface (1,92%), and web application integrated with data analytic (1,92%).
Table 6. Outcomes of type of system.
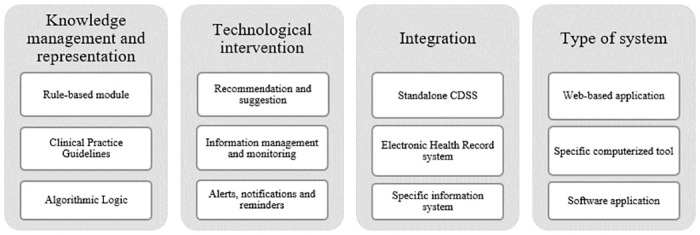
The outcomes for each group were varied regarding the type of the CDSS, knowledge management, integration of systems and the technological features. Despite this diversity, we were able to notice a trend in results based on their frequent presence in the reviewed studies. As shown in Fig 3 , the most common knowledge representation and management techniques were the rule-based module, clinical practice guidelines and algorithmic logic. Regarding to the technological intervention or feature, the three top trends were recommendation and suggestion, information management and monitoring, and alerts, notifications and reminders. Standalone CDSS were the most common one, following integration with Electronic Healthcare Records (EHR) systems and specific healthcare information systems. The most frequent type of system were, respectively, web-based application, specific computerized tool, and software application. Despite the individual results, we also noticed a trend towards a combination of results. The most frequent sequence related to knowledge management was the mix of algorithmic logic with clinical practice guidelines. For technological features, the most common combination was the joining of recommendation and suggestion with alerts, notifications and reminders.
Fig 3. Characterization of general trends obtained through the review.
Temporal evolution
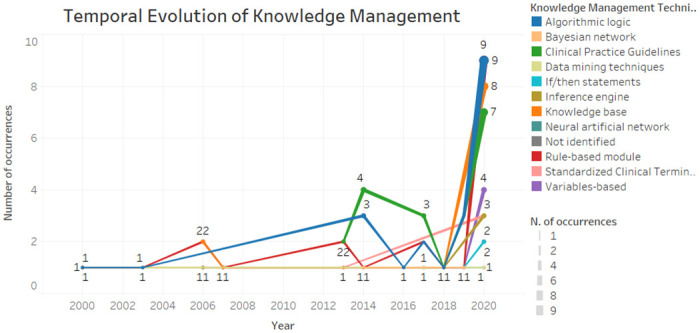
Fig 4 demonstrates the temporal evolution of knowledge management and representation techniques used in studies. It is possible to verify that rule-based module and the algorithmic logic have been present since 2000 until today, mostly. As for clinical practice guidelines, on the other hand, proved to be a current issue due to their presence from 2013 to 2020. In general, the characteristics remain in existence over time, with a greater occurrence in 2020 due to the number of articles analysed that year. In contrast, the characteristics of the Bayesian network, if / then statements and neural networks were considered more recent, emerging from 2014.
Fig 4. Temporal evolution of knowledge management.
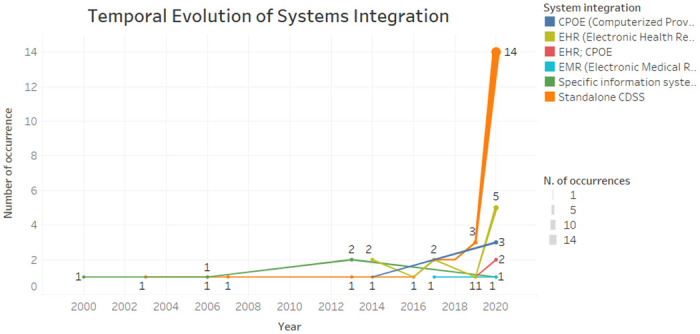
According to Fig 5 , we can see that the integration of standalone CDSS has been increasing in recent years. The integration with EHR systems, oscillated between 2014 to 2020, standing out also in the last year. Specific health information systems have been integrated into the CDSS from 2000 to 2020, on a regular basis.
Fig 5. Temporal evolution of system integration.
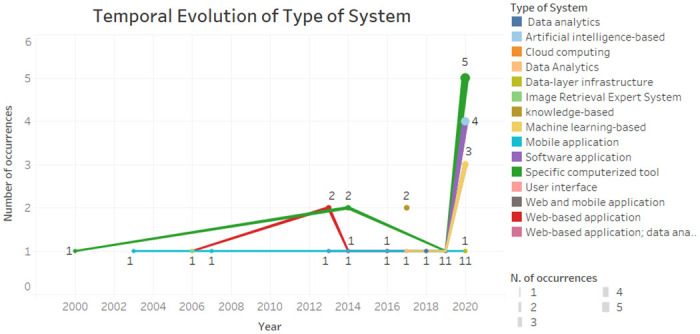
According to Fig 6 , the specific computerized tools have been present since the beginning of the time interval and were more present in the year 2020. The software application, machine learning-based, was already present. The mobile application remained constant. Web-based application was more prevalent in 2013.
Fig 6. Temporal evolution of type of system.
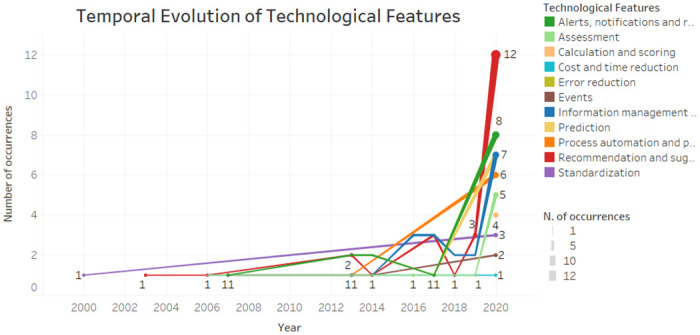
As showed in Fig 7 the alerts, notifications and reminders and the information management and monitoring feature were more present in 2020, but also present in some previous year. The recommendations and suggestions feature was present from 2003 to 2020. Standardization was present in 2000 and only returned in 2020. The calculation and scoring, cost and time reduction and prediction features were considered the most recent ones.
Fig 7. Temporal evolution of CDSS features.
Maturity staging model
For a CDSS to be considered effective, it has to reach the prestigious level of maturity. Thus, it is important to recognize the degree of maturity associated with the characterization of the CDSSs. In order to complement the study, a cross-checking was made with the trends characteristics facing the four phases of Simon’s decision-making theory [ 8 ]. Thus, four stages were classified aiming to represent the level of maturity of a system, based on Simon’s phases:
Stage 4: Implementation + Choice + Design + Intelligence
Stage 3: Choice + Design + Intelligence
Stage 2: Design + Intelligence
Stage 1: Intelligence
Table 7 represents the crossing of the Simon’s phases with the three most common characteristics of each group, in order to assess their stage of maturity. The columns referring to the maturity stages, correspond to the number of articles of the respective characteristic, given their presence in each Simon phase. It should be noted that the values are cumulative, that is, for a stage to be reached, it must contain the previous stage. All studies corresponding to each characteristic are present in the initial phase of Intelligence. Maturity is calculated using the weighted average (WAVG), which should vary between 1 and 4, corresponding to each stage.
Table 7. Maturity Staging Model applied on the reviewed studies.
Main findings.
This article aimed to develop a systematic review within the scope of Clinical Decision Support Systems (CDSS). The first research question (RQ1) was to identify the tendency of adopted approaches in CDSS development addressed in the reviewed studies, as shown in Fig 3 . To classify the revised studies, information about four predefined groups were extracted: knowledge management, technological intervention, system integration and type of system. Although there are other characteristics associated with a CDSS, the authors chose to extract specific information that go beyond the main objective of a CDSS, which is to assist the decision maker in the decision-making process. The results showed that the most used techniques for the knowledge representation in the systems are the creation of modules based on rules, clinical practice guidelines and logic algorithms. The technological features most present in CDSS are recommendation and suggestion, monitoring and information management, and alerts and reminders. Usually, CDSS are used standalone, following the integration with Electronic Health Systems or a specific information system. It was also identified that the most common types of systems are web applications, specific computer tools, and software applications.
In order to answer the second research question (RQ2), a preliminary classification during the review was carried out identifying which phase of the decision-making process model the CDSS was in. According to Simon’s bounded rationality theory [ 8 ], it was considered: Intelligence phase as responsible for analysis, exploration and description of the problem to be faced; the Design phase as the development and analysis of possible solutions to the problem at hand; in the Choice phase, the most appropriate solution is chosen to solve the problem; the Implementation seeks to apply the solution to the problem in question.
Regarding the maturity staging model proposed in Table 7 , when the stage 4 is achieved, the system has reached its prestigious level of maturity. However, this is not verified in the analysis. The weighted average (WAVG) of the characteristics showed very similar values, meaning that the CDSS of the reviewed studies predominates in Simon’s Intelligence and Design phases, equivalent to stage 2 of maturity. The recommendation and suggestion (1.55) and software application (1.53) characteristics have the lowest values nearly reaching the previous phase of Intelligence. The algorithmic logic characteristic stands out the most and shows that it is closer to achieving the upper stage of maturity (stage 3), meeting the Choice phase.
There are other systematic reviews that study the role of CDSS. However, to the best of our knowledge, existing studies analyse CDSS applied to a specific clinical context and do not evaluate its effectiveness as a whole (as [ 11 , 12 , 71 ]). The present systematic review selected a set of studies that approach the development of a CDSS characterizing them in order to trace a global trend, as well as assess their level of maturity. In this sense, it was possible to analyse the CDSS in its completeness, covering different approaches, techniques and purposes of use. The main contribution of this work was the proposed maturity staging model, that allowed to identify a gap between the state-of-art and the desirable stage of maturity in order to provide an effective CDSS. The results showed that the revised CDSS do not go beyond stage 2, meaning that CDSS are not succeeding in the healthcare arena due to the lack of maturity, i.e., as CDSS are not capable of supporting the choice of actions in clinical settings and are also not involved in the implementation of these actions.
This study allowed to raise a concern in the development of CDSS and to raise awareness that limited systems are being created and that may be far from being optimized. The projection of this study allowed us to portray a reality of many decision support systems in the health area, demonstrating the opposite of what it should be. When a system reaches the model implementation phase, it should be closer to reaching its optimization and not going back to the previous phases. This immaturity may be due to a lack of understanding of the real problem, difficulty in choosing the ideal solution, and failures in usability tests. An in-depth analysis must be done to discover the main constraints that prevent the inclusion of the decision-making phases in the CDSS.
Limitations and future research
The maturity staging model, combined with the phases of the decision-making process, serves to assess the effectiveness of the decision. When an implemented decision does not produce the desired results, there are likely to be several causes, such as incorrect problem definition, poor evaluation of alternatives, or inadequate implementation [ 18 ]. The proposed alternative may not be successful, which will lead to a new analysis of the problem, evaluation of alternatives and selection of a new alternative. Thus, evaluation is a key factor in the process because decision-making is a continuous and never-ending process.
Some limitations should be stressed. First, we were unable to perform a meta-analysis due to the variation in the type of studies analysed, as well as to use a quality assessment methodology to assess the quality of studies. The sample number of eligible studies was also limited. Another limitation was regarding the characterization of the CDSS, due to some studies not directly explaining the respective approach used. There may also be some inconsistency in the review carried out due to the personal opinions of each reviewer.
It is known that in the clinical area regulations and ethical issues (e.g. computer based control of infusion pumps) limit the application of the most advanced phases of the decision-making process (e.g. choice and implementation). This means that the highest value for the maturity stage might be lower than 4. Thus, some difficulties encountered in the adoption of CDSS may be related to the medical context, such as: the development of methods for supporting choice phase of decision making; interoperability among medical devices and health care information systems; technology acceptance; ethical and regulatory restrictions; poor involvement of professionals and organizations. A lot of work should be done in the future to mitigate those limitations, starting with: i) consider a larger sample of studies; ii) determine the accepted range of values for the maturity stage in particular clinical context of application; and iii) depict what it should be the focus of research in order to fulfil the actual maturity gap and develop effective CDSS.
Clinical Decision Support Systems (CDSS) represent the decision support activity and can be translated into a machine-readable computerized format. Most healthcare information systems are encouraged to or already include clinical decision support practices to organize clinical knowledge and improve the decision-making process [ 3 ]. In order to answer the research questions formulated in this work, a systematic review was developed to identify the techniques and approaches used in CDSS from 52 studies. The outcomes were varied and did not show a main pattern, leading to limited evidence. Nevertheless, it was identified the top three trends of the four defined groups: knowledge management, technological features, type of system, and system integration. In addition, this study allowed a more complete analysis to understand the state of maturity of the CDSS. A crossover of the identified trends was made to identify the maturity regarding the four phases proposed by Simon. The results demonstrated the lack of maturity of the CDSS presented by the reviewed studies.
Decision making is a process of making a choice between several alternatives to achieve a desired outcome. Among these possible causes, the most common and serious error is an inadequate definition of the problem. When the problem is not defined correctly, the alternative selected and implemented will not produce the desired result. That said, we believe that the biggest difficulties on the CDSS adoption are in the operational clinical environment, with the involvement of characteristics, processes, and stakeholders. Furthermore, based on the identified gap in this study, an agenda should be created for what an effective CDSS should be. There is an incentive for the scientific community to contribute to mitigate the limitations in order to achieve more effective Clinical Decision Support Systems.
Supporting information
Data availability.
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
This study was supported by FCT – Fundação para a Ciência e Tecnologia, within the scope of the project DSAIPA/DS/0084/2018, FH work was supported by the grant 2021.06230.BD. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
- 1. Salazar M, Duarte J, Pereira R, Portela F, Abelha A, Machado J, et al. Step towards Paper Free Hospital through Electronic Health Record. 2013;747:685–694. [ Google Scholar ]
- 2. Friedman CP. A “Fundamental Theorem” of Biomedical Informatics. Journal of the American Medical Informatics Association. 2009;16(2):169–170. doi: 10.1197/jamia.M3092 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 3. Handler T, Holtmeier R, Metzger J, Overhage M, Taylor S, Underwood C. HIMSS electronic health record definitional model. 2003;(January 2003):0–8.
- 4. Murty KG, Liu J, Wan Yw, Linn R. A decision support system for quayside operations in a container terminal. Decision Support Systems. 2005;59(1):312–324. [ Google Scholar ]
- 5. Vasconcelos J, Rocha A, Gomes R. Sistemas de Informação de Apoio à Decisão Clínica: Estudo de um caso de uma Instituição de Saúde. 2004.
- 6. Neves J, Santos M, Machado J, Abelha A, Allegro S, Salazar M. Electronic Health Records and Decision Support Local and Global Perspectives. WSEAS TRANSACTIONS on BIOLOGY and BIOMEDICINE. 2008;5(8):189–198. [ Google Scholar ]
- 7. Kalantari B. Herbert A. Simon on making decisions: Enduring insights and bounded rationality. Journal of Management History. 2010;16:509–520. doi: 10.1108/17511341011073988 [ DOI ] [ Google Scholar ]
- 8. Simon HA. The New Science of Management Decision. USA: Prentice Hall PTR; 1960. [ Google Scholar ]
- 9. Sprague RH, Carlson ED. Building Effective Decision Support Systems. Prentice Hall Professional Technical Reference; 1982.
- 10. Bayoumi I, Balas MA, Handler SM, Dolovich L, Hutchison B, Holbrook A. The effectiveness of computerized drug-lab alerts: A systematic review and meta-analysis. International Journal of Medical Informatics. 2014;83(6):406–415. doi: 10.1016/j.ijmedinf.2014.03.003 [ DOI ] [ PubMed ] [ Google Scholar ]
- 11. Jia P, Zhang L, Chen J, Zhao P, Zhang M. The effects of clinical decision support systems on medication safety: An overview. PLoS ONE. 2016;11. doi: 10.1371/journal.pone.0167683 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 12. Curtis CE, Bahar FA, Marriott JF. The effectiveness of computerised decision support on antibiotic use in hospitals: A systematic review. PLoS ONE. 2017;12. doi: 10.1371/journal.pone.0183062 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 13. Turpin S, Marais M. Decision Making: Theory and Practice. The Journal of Finance. 2004;29(4):1341. [ Google Scholar ]
- 14. Turban E, Delen D, Sharda R. Business intelligence and analytics; 2014.
- 15. Moher D, Liberati A, Tetzlaff J, Altman DG, Altman D, Antes G, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Medicine. 2009;6(7). doi: 10.1371/journal.pmed.1000097 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 16. Scimago. Scimago Journal and Country Rank, year = 2022, url = https://www.scimagojr.com/ .
- 17. Kock E. A Broader Perspective to Decision Support Systems. 2003.
- 18. Lunenburg FC. THE DECISION MAKING PROCESS; 2010.
- 19. Falciglia GH, Murthy K, Holl JL, Palac HL, Woods DM, Robinson DT. Low prevalence of clinical decision support to calculate caloric and fluid intake for infants in the neonatal intensive care unit. Journal of Perinatology. 2020;40(3):497–503. doi: 10.1038/s41372-019-0546-z [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 20. Lee K, Lee SH. Artificial intelligence-driven oncology clinical decision support system for multidisciplinary teams. Sensors (Switzerland). 2020;20(17):1–12. doi: 10.3390/s20174693 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 21. Catalani C, Green E, Owiti P, Keny A, Diero L, Yeung A, et al. A clinical decision support system for integrating tuberculosis and HIV care in Kenya: A human-centered design approach. PLoS ONE. 2014;9(8). doi: 10.1371/journal.pone.0103205 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 22. Akhloufi H, Verhaegh SJC, Jaspers MWM, Melles DC, van der Sijs H, Verbon A. A usability study to improve a clinical decision support system for the prescription of antibiotic drugs. PLoS ONE. 2019;14(9):1–14. doi: 10.1371/journal.pone.0223073 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 23. Tamburrano A, Vallone D, Carrozza C, Urbani A, Sanguinetti M, Nicolotti N, et al. Evaluation and cost estimation of laboratory test overuse in 43 commonly ordered parameters through a Computerized Clinical Decision Support System (CCDSS) in a large university hospital. PLoS ONE. 2020;15(8 August):1–11. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 24. Kim HJ, Kim HJ, Park Y, Lee WS, Lim Y, Kim JH. Clinical Genome Data Model (cGDM) provides Interactive Clinical Decision Support for Precision Medicine. Scientific Reports. 2020;10(1):1–13. doi: 10.1038/s41598-020-58088-2 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 25. Lin L, Hu PJH, Liu Sheng OR. A decision support system for lower back pain diagnosis: Uncertainty management and clinical evaluations. Decision Support Systems. 2006;42(2):1152–1169. doi: 10.1016/j.dss.2005.10.007 [ DOI ] [ Google Scholar ]
- 26. Rao GR, Turoff M. Hypermedia-based group decision support system to support collaborative medical decision-making. Decision Support Systems. 2000;30(2):187–216. doi: 10.1016/S0167-9236(00)00096-8 [ DOI ] [ Google Scholar ]
- 27. Van Valkenhoef G, Tervonen T, Zwinkels T, De Brock B, Hillege H. ADDIS: A decision support system for evidence-based medicine. Decision Support Systems. 2013;55(2):459–475. doi: 10.1016/j.dss.2012.10.005 [ DOI ] [ Google Scholar ]
- 28. Hu PJH, Wei CP, Liu Sheng OR. Evaluating a decision support system for patient image pre-fetching: An experimental study. Decision Support Systems. 2006;42(3):1730–1746. doi: 10.1016/j.dss.2006.02.016 [ DOI ] [ Google Scholar ]
- 29. Michalowski W, Rubin S, Slowinski R, Wilk S. Mobile clinical support system for pediatric emergencies. Decision Support Systems. 2003;36(2):161–176. doi: 10.1016/S0167-9236(02)00140-9 [ DOI ] [ Google Scholar ]
- 30. Yao W, Kumar A. CONFlexFlow: Integrating Flexible clinical pathways into clinical decision support systems using context and rules. Decision Support Systems. 2013;55(2):499–515. doi: 10.1016/j.dss.2012.10.008 [ DOI ] [ Google Scholar ]
- 31. Lussier YA, Williams R, Li J, Jalan S, Borlawsky T, Stern E, et al. Partitioning knowledge bases between advanced notification and clinical decision support systems. Decision Support Systems. 2007;43(4):1274–1286. doi: 10.1016/j.dss.2006.02.006 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 32. Corny J, Rajkumar A, Martin O, Dode X, Lajonchère JP, Billuart O, et al. A machine learning–based clinical decision support system to identify prescriptions with a high risk of medication error. Journal of the American Medical Informatics Association. 2020;00(0):1–7. doi: 10.1093/jamia/ocaa154 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 33. Altay EV, Alatas B. A novel clinical decision support system for liver fibrosis using evolutionary multi-objective method based numerical association analysis. Medical Hypotheses. 2020;144(May):110028. doi: 10.1016/j.mehy.2020.110028 [ DOI ] [ PubMed ] [ Google Scholar ]
- 34. Yoo J, Lee J, Rhee PL, Chang DK, Kang M, Choi JS, et al. Alert Override Patterns with a Medication Clinical Decision Support System in an Academic Emergency Department: Retrospective Descriptive Study (Preprint). JMIR Medical Informatics. 2020;8:1–14. doi: 10.2196/23351 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 35. Hamedan F, Orooji A, Sanadgol H, Sheikhtaheri A. Clinical decision support system to predict chronic kidney disease: A fuzzy expert system approach. International Journal of Medical Informatics. 2020;138(December 2019):104134. doi: 10.1016/j.ijmedinf.2020.104134 [ DOI ] [ PubMed ] [ Google Scholar ]
- 36. Holmström IK, Kaminsky E, Lindberg Y, Spangler D, Winblad U. Registered Nurses’ experiences of using a clinical decision support system for triage of emergency calls: A qualitative interview study. Journal of Advanced Nursing. 2020;76(11):3104–3112. doi: 10.1111/jan.14542 [ DOI ] [ PubMed ] [ Google Scholar ]
- 37. Chin DL, Wilson MH, Trask AS, Johnson VT, Neaves BI, Gojova A, et al. Repurposing Clinical Decision Support System Data to Measure Dosing Errors and Clinician-Level Quality of Care. Journal of Medical Systems. 2020;44(10). doi: 10.1007/s10916-020-01603-9 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 38. Niazkhani Z, Fereidoni M, Rashidi Khazaee P, Shiva A, Makhdoomi K, Georgiou A, et al. Translation of evidence into kidney transplant clinical practice: Managing drug-lab interactions by a context-aware clinical decision support system. BMC Medical Informatics and Decision Making. 2020;20(1):1–13. doi: 10.1186/s12911-020-01196-w [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 39. Berge GT, Granmo OC, Tveit TO. Combining unsupervised, supervised, and rule-based algorithms for text mining of electronic health records: A clinical decision support system for identifying and classifying allergies of concern for anesthesia during surgery. Information Systems Development: Advances in Methods, Tools and Management—Proceedings of the 26th International Conference on Information Systems Development, ISD 2017. 2017.
- 40. Wasylewicz ATM, Scheepers-Hoeks AMJW. Clinical decision support systems. Fundamentals of Clinical Data Science. 2018;(June):153–169.
- 41. Smit K, Koornneef P, Nysingh J, van Zwienen M, Berkhout M, Ravesteyn P. Transforming clinical practice guideline usage through the use of a clinical decision support system: An explorative study at the university medical centre Utrecht. 30th Bled eConference: Digital Transformation—From Connecting Things to Transforming our Lives, BLED 2017. 2017; p. 577–592.
- 42. Dalaba MA, Akweongo P, Williams J, Saronga HP, Tonchev P, Sauerborn R, et al. Costs associated with implementation of computer-assisted clinical decision support system for antenatal and delivery care: Case study of Kassena-Nankana district of northern Ghana. PLoS ONE. 2014;9(9). doi: 10.1371/journal.pone.0106416 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 43. Sim LLW, Ban KHK, Tan TW, Sethi SK, Loh TP. Development of a clinical decision support system for diabetes care: A pilot study. PLoS ONE. 2017;12(2):1–15. doi: 10.1371/journal.pone.0173021 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 44. Bernasconi A, Crabbé F, Adedeji AM, Bello A, Schmitz T, Landi M, et al. Results from one-year use of an electronic Clinical Decision Support System in a post-conflict context: An implementation research. PLoS ONE. 2019;14(12):1–17. doi: 10.1371/journal.pone.0225634 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 45. Menon S, Tarrago R, Carlin K, Wu H, Yonekawa K. Impact of integrated clinical decision support systems in the management of pediatric acute kidney injury: a pilot study. Pediatric Research. 2020;(April):1–7. [ DOI ] [ PubMed ] [ Google Scholar ]
- 46. Arain Y, Banda JM, Faulkenberry J, Bhutani VK, Palma JP. Clinical decision support tool for phototherapy initiation in preterm infants. Journal of Perinatology. 2020;40(10):1518–1523. doi: 10.1038/s41372-020-00782-0 [ DOI ] [ PubMed ] [ Google Scholar ]
- 47. Séroussi B, Laouénan C, Gligorov J, Uzan S, Mentré F, Bouaud J. Which breast cancer decisions remain non-compliant with guidelines despite the use of computerised decision support? British Journal of Cancer. 2013;109(5):1147–1156. doi: 10.1038/bjc.2013.453 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 48. Akçura MT, Ozdemir ZD. Drug prescription behavior and decision support systems. Decision Support Systems. 2014;57(1):395–405. [ Google Scholar ]
- 49. Johnson MP, Zheng K, Padman R. Modeling the longitudinality of user acceptance of technology with an evidence-adaptive clinical decision support system. Decision Support Systems. 2014;57(1):444–453. doi: 10.1016/j.dss.2012.10.049 [ DOI ] [ Google Scholar ]
- 50. Lam Shin Cheung J, Paolucci N, Price C, Sykes J, Gupta S. A system uptake analysis and GUIDES checklist evaluation of the Electronic Asthma Management System: A point-of-care computerized clinical decision support system. Journal of the American Medical Informatics Association. 2020;27(5):726–737. doi: 10.1093/jamia/ocaa019 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 51. Downie AS, Hancock M, Abdel Shaheed C, McLachlan AJ, Kocaballi AB, Williams CM, et al. An Electronic Clinical Decision Support System for the Management of Low Back Pain in Community Pharmacy: Development and Mixed Methods Feasibility Study. JMIR Medical Informatics. 2020;8(5):e17203. doi: 10.2196/17203 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 52. Xu F, Sepúlveda MJ, Jiang Z, Wang H, Li J, Liu Z, et al. Effect of an Artificial Intelligence Clinical Decision Support System on Treatment Decisions for Complex Breast Cancer. JCO Clinical Cancer Informatics. 2020;(4):824–838. doi: 10.1200/CCI.20.00018 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 53. Torres Silva EA, Uribe S, Smith J, Luna Gomez IF, Florez-Arango JF. XML Data and Knowledge-Encoding Structure for a Web-Based and Mobile Antenatal Clinical Decision Support System: Development Study. JMIR Formative Research. 2020;4(10):e17512. doi: 10.2196/17512 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 54. Miller MK, Mollen C, Behr K, Dowd MD, Miller E, Satterwhite CL, et al. Development of a Novel Computerized Clinical Decision Support System to Improve Adolescent Sexual Health Care Provision. Academic Emergency Medicine. 2019;26(4):420–433. doi: 10.1111/acem.13570 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 55. Bond SE, Chubaty AJ, Adhikari S, Miyakis S, Boutlis CS, Yeo WW, et al. Outcomes of multisite antimicrobial stewardship programme implementation with a shared clinical decision support system. Journal of Antimicrobial Chemotherapy. 2017;72(7):2110–2118. doi: 10.1093/jac/dkx080 [ DOI ] [ PubMed ] [ Google Scholar ]
- 56. Strockbine VL, Gehrie EA, Zhou QP, Guzzetta CE. Reducing Unnecessary Phlebotomy Testing Using a Clinical Decision Support System. Journal for healthcare quality: official publication of the National Association for Healthcare Quality. 2020;42(2):98–105. doi: 10.1097/JHQ.0000000000000245 [ DOI ] [ PubMed ] [ Google Scholar ]
- 57. Jiang X, Wells A, Brufsky A, Neapolitan R. A clinical decision support system learned from data to personalize treatment recommendations towards preventing breast cancer metastasis. PLoS ONE. 2019;14(3):1–18. doi: 10.1371/journal.pone.0213292 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 58. De Lima Marinho C, Maioli MCP, Do Amaral JLM, Lopes AJ, De Melo PL. Respiratory resistance and reactance in adults with sickle cell anemia: Part 2—Fractional-order modeling and a clinical decision support system for the diagnosis of respiratory disorders. PLoS ONE. 2019;14(3):1–21. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 59. Wu G, Yang P, Xie Y, Woodruff HC, Rao X, Guiot J, et al. Development of a clinical decision support system for severity risk prediction and triage of COVID-19 patients at hospital admission: An international multicentre study. European Respiratory Journal. 2020;56(2). doi: 10.1183/13993003.01104-2020 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 60. Choi GH, Yun J, Choi J, Lee D, Shim JH, Lee HC, et al. Development of machine learning-based clinical decision support system for hepatocellular carcinoma. Scientific Reports. 2020;10(1):1–10. doi: 10.1038/s41598-020-71796-z [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 61. Lichtenegger KM, Aberer F, Tuca AC, Donsa K, Höll B, Schaupp L, et al. Safe and Sufficient Glycemic Control by Using a Digital Clinical Decision Support System for Patients With Type 2 Diabetes in a Routine Setting on General Hospital Wards. Journal of Diabetes Science and Technology. 2020. [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 62. Vargason T, Cohn J, Schultz O. The Open Repository @ Binghamton (The ORB) A Clinical Decision Support System for Malignant Pleural Effusion Analysis. 2016.
- 63. Xu B, Li C, Zhuang H, Wang J, Wang Q, Wang C, et al. Distributed gene clinical decision support system based on cloud computing. BMC Medical Genomics. 2018;11. doi: 10.1186/s12920-018-0415-1 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 64. Schaaf J, Sedlmayr M, Prokosch HU, Ganslandt T, Schade-Brittinger C, Wagner MV, et al. The status quo of rare diseases centres for the development of a clinical decision support system—A cross-sectional study. Studies in Health Technology and Informatics. 2020;271:176–183. [ DOI ] [ PubMed ] [ Google Scholar ]
- 65. Xu Q, Zhai JC, Huo CQ, Li Y, Dong XJ, Li DF, et al. OncoPDSS: An evidence-based clinical decision support system for oncology pharmacotherapy at the individual level. BMC Cancer. 2020;20(1):1–10. doi: 10.1186/s12885-020-07221-5 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 66. Berrouiguet S, Barrigón ML, Brandt SA, Ovejero-García S, Álvarez-García R, Carballo JJ, et al. Development of a web-based clinical decision support system for drug prescription: Non-interventional naturalistic description of the antipsychotic prescription patterns in 4345 outpatients and future applications. PLoS ONE. 2016;11(10):1–16. doi: 10.1371/journal.pone.0163796 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 67. Piri S, Delen D, Liu T, Zolbanin HM. A data analytics approach to building a clinical decision support system for diabetic retinopathy: Developing and deploying a model ensemble. Decision Support Systems. 2017;101:12–27. doi: 10.1016/j.dss.2017.05.012 [ DOI ] [ Google Scholar ]
- 68. Piri S. Missing care: A framework to address the issue of frequent missing values;The case of a clinical decision support system for Parkinson’s disease. Decision Support Systems. 2020;136(November 2019):113339. doi: 10.1016/j.dss.2020.113339 [ DOI ] [ Google Scholar ]
- 69. Breitbart EW, Choudhury K, Andersen AD, Bunde H, Breitbart M, Sideri AM, et al. Improved patient satisfaction and diagnostic accuracy in skin diseases with a Visual Clinical Decision Support System-A feasibility study with general practitioners. PLoS ONE. 2020;15(7 July):1–12. doi: 10.1371/journal.pone.0235410 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
- 70. Ucuz I, Ciçek AU, Ari A, Ozcan OO, Aybüke Sari S. Determining the probability of juvenile delinquency by using support vector machines and designing a clinical decision support system. Medical Hypotheses. 2020;143(July):110118. doi: 10.1016/j.mehy.2020.110118 [ DOI ] [ PubMed ] [ Google Scholar ]
- 71. Anchala R, Pinto MP, Shroufi A, Chowdhury R, Sanderson J, Johnson L, et al. The Role of Decision Support System (DSS) in Prevention of Cardiovascular Disease: A Systematic Review and Meta-Analysis. PLoS ONE. 2012;7. doi: 10.1371/journal.pone.0047064 [ DOI ] [ PMC free article ] [ PubMed ] [ Google Scholar ]
Decision Letter 0
Gabriele oliva.
22 Mar 2022
PONE-D-20-38355Towards Effective Clinical Decision Support Systems: A Systematic ReviewPLOS ONE
Dear Dr. Hak,
Thank you for submitting your manuscript to PLOS ONE. After careful consideration, we feel that it has merit but does not fully meet PLOS ONE’s publication criteria as it currently stands. Therefore, we invite you to submit a revised version of the manuscript that addresses the points raised during the review process.
Please submit your revised manuscript by May 06 2022 11:59PM. If you will need more time than this to complete your revisions, please reply to this message or contact the journal office at [email protected] . When you're ready to submit your revision, log on to https://www.editorialmanager.com/pone/ and select the 'Submissions Needing Revision' folder to locate your manuscript file.
Please include the following items when submitting your revised manuscript:
A rebuttal letter that responds to each point raised by the academic editor and reviewer(s). You should upload this letter as a separate file labeled 'Response to Reviewers'.
A marked-up copy of your manuscript that highlights changes made to the original version. You should upload this as a separate file labeled 'Revised Manuscript with Track Changes'.
An unmarked version of your revised paper without tracked changes. You should upload this as a separate file labeled 'Manuscript'.
If you would like to make changes to your financial disclosure, please include your updated statement in your cover letter. Guidelines for resubmitting your figure files are available below the reviewer comments at the end of this letter.
If applicable, we recommend that you deposit your laboratory protocols in protocols.io to enhance the reproducibility of your results. Protocols.io assigns your protocol its own identifier (DOI) so that it can be cited independently in the future. For instructions see: https://journals.plos.org/plosone/s/submission-guidelines#loc-laboratory-protocols . Additionally, PLOS ONE offers an option for publishing peer-reviewed Lab Protocol articles, which describe protocols hosted on protocols.io. Read more information on sharing protocols at https://plos.org/protocols?utm_medium=editorial-email&utm_source=authorletters&utm_campaign=protocols .
We look forward to receiving your revised manuscript.
Kind regards,
Gabriele Oliva, Ph.D
Academic Editor
Journal Requirements:
When submitting your revision, we need you to address these additional requirements.
1. Please ensure that your manuscript meets PLOS ONE's style requirements, including those for file naming. The PLOS ONE style templates can be found at
https://journals.plos.org/plosone/s/file?id=wjVg/PLOSOne_formatting_sample_main_body.pdf and
https://journals.plos.org/plosone/s/file?id=ba62/PLOSOne_formatting_sample_title_authors_affiliations.pdf
2. We note that the grant information you provided in the ‘Funding Information’ and ‘Financial Disclosure’ sections do not match.
When you resubmit, please ensure that you provide the correct grant numbers for the awards you received for your study in the ‘Funding Information’ section.
3. In your Data Availability statement, you have not specified where the minimal data set underlying the results described in your manuscript can be found. PLOS defines a study's minimal data set as the underlying data used to reach the conclusions drawn in the manuscript and any additional data required to replicate the reported study findings in their entirety. All PLOS journals require that the minimal data set be made fully available. For more information about our data policy, please see http://journals.plos.org/plosone/s/data-availability .
Upon re-submitting your revised manuscript, please upload your study’s minimal underlying data set as either Supporting Information files or to a stable, public repository and include the relevant URLs, DOIs, or accession numbers within your revised cover letter. For a list of acceptable repositories, please see http://journals.plos.org/plosone/s/data-availability#loc-recommended-repositories . Any potentially identifying patient information must be fully anonymized.
Important: If there are ethical or legal restrictions to sharing your data publicly, please explain these restrictions in detail. Please see our guidelines for more information on what we consider unacceptable restrictions to publicly sharing data: http://journals.plos.org/plosone/s/data-availability#loc-unacceptable-data-access-restrictions . Note that it is not acceptable for the authors to be the sole named individuals responsible for ensuring data access.
We will update your Data Availability statement to reflect the information you provide in your cover letter.
4. Please upload a new copy of Figures 1 and 2 as the detail is not clear. Please follow the link for more information: https://blogs.plos.org/plos/2019/06/looking-good-tips-for-creating-your-plos-figures-graphics/ " https://blogs.plos.org/plos/2019/06/looking-good-tips-for-creating-your-plos-figures-graphics/
5. We note you have included a table to which you do not refer in the text of your manuscript. Please ensure that you refer to Tables 1, 2, 3, 4 and 5 in your text; if accepted, production will need this reference to link the reader to the Table.
6. Please review your reference list to ensure that it is complete and correct. If you have cited papers that have been retracted, please include the rationale for doing so in the manuscript text, or remove these references and replace them with relevant current references. Any changes to the reference list should be mentioned in the rebuttal letter that accompanies your revised manuscript. If you need to cite a retracted article, indicate the article’s retracted status in the References list and also include a citation and full reference for the retraction notice.
Additional Editor Comments (if provided):
Two reviews were obtained, both suggesting minor edits to the paper. I agree with the reviewers' evaluation and I am recommending a minor revision of the paper.
[Note: HTML markup is below. Please do not edit.]
Reviewers' comments:
Reviewer's Responses to Questions
Comments to the Author
1. Is the manuscript technically sound, and do the data support the conclusions?
The manuscript must describe a technically sound piece of scientific research with data that supports the conclusions. Experiments must have been conducted rigorously, with appropriate controls, replication, and sample sizes. The conclusions must be drawn appropriately based on the data presented.
Reviewer #1: Partly
Reviewer #2: Partly
2. Has the statistical analysis been performed appropriately and rigorously?
Reviewer #1: I Don't Know
Reviewer #2: I Don't Know
3. Have the authors made all data underlying the findings in their manuscript fully available?
The PLOS Data policy requires authors to make all data underlying the findings described in their manuscript fully available without restriction, with rare exception (please refer to the Data Availability Statement in the manuscript PDF file). The data should be provided as part of the manuscript or its supporting information, or deposited to a public repository. For example, in addition to summary statistics, the data points behind means, medians and variance measures should be available. If there are restrictions on publicly sharing data—e.g. participant privacy or use of data from a third party—those must be specified.
Reviewer #1: Yes
Reviewer #2: Yes
4. Is the manuscript presented in an intelligible fashion and written in standard English?
PLOS ONE does not copyedit accepted manuscripts, so the language in submitted articles must be clear, correct, and unambiguous. Any typographical or grammatical errors should be corrected at revision, so please note any specific errors here.
5. Review Comments to the Author
Please use the space provided to explain your answers to the questions above. You may also include additional comments for the author, including concerns about dual publication, research ethics, or publication ethics. (Please upload your review as an attachment if it exceeds 20,000 characters)
Reviewer #1: The topic is quite relevant and important. Overall, the paper achieved its overall aim i.e. " to systematically review the extant empirical evidence on this topic, establish the status quo of the research, and propose an agenda for further studies" However, there are a number of issues with the methods and the reporting as documented below.
This paper provides an extensive literature review related to the key features that influences the development of effective CDSS.
• The authors argue that focused only on papers published in five online data sources. There are other resources such as valuable conferences that might be even more relevant to the study's purpose than those in grey literature.
• The search strategy is not comprehensive enough for a systematic review. The search strategy is apparently missing.
• In the information resources, it is stated that five online data sources that have a major impact on health information systems research were selected. On what basis are these five main sources (references)? Which reference (s)?
Discussion:
• The authors argue that none of the reviews have provided a global trend of a CDSS through a systematic review, but the reader might find this claim ambitious (Given that you have not searched the database anymore).
• In the discussion, in systematic review articles, the results of the article should be compared with other articles related to this paper.
The structuring of the discussion and conclusion presentation is not well-argued and not clear to me.
Please rewrite the conclusion. Start with the main findings which should contain an overview of the literature you studied.
Reviewer #2: The article carries out a systematic review of clinical decision support systems. In particular, they want to identify the articles dealing with this issue by highlighting the characteristics that distinguish them, also providing information on the purpose and the recipient.
Five open source online resources are considered. In addition, defined a methodology based on specific criteria: freely accessible articles, written in English, must provide medical support, media must be computer or technological. Two reviewers will analyze the title, abstract and keyword of the articles according to the parameters and will extract the most significant ones. The extracted elements will be read entirely and analyzed. In particular, four main features will be analyzed: the management and technical representations, technological characteristics of the system, the type of system and the integration of the system. The data were classified without providing an analysis of the analyzed characteristics and differences between the various solutions proposed. It also shows the trend of the distribution and the use of different methodologies not analyzing the data or providing a comment of the same.
Authors should express more clearly the choice of criteria and the analysis made. In addition, the differences and aspects highlighted by the collection should be highlighted and clarify.
6. PLOS authors have the option to publish the peer review history of their article ( what does this mean? ). If published, this will include your full peer review and any attached files.
If you choose “no”, your identity will remain anonymous but your review may still be made public.
Do you want your identity to be public for this peer review? For information about this choice, including consent withdrawal, please see our Privacy Policy .
Reviewer #1: Yes: Maryam Zahmatkeshan
Reviewer #2: No
[NOTE: If reviewer comments were submitted as an attachment file, they will be attached to this email and accessible via the submission site. Please log into your account, locate the manuscript record, and check for the action link "View Attachments". If this link does not appear, there are no attachment files.]
While revising your submission, please upload your figure files to the Preflight Analysis and Conversion Engine (PACE) digital diagnostic tool, https://pacev2.apexcovantage.com/ . PACE helps ensure that figures meet PLOS requirements. To use PACE, you must first register as a user. Registration is free. Then, login and navigate to the UPLOAD tab, where you will find detailed instructions on how to use the tool. If you encounter any issues or have any questions when using PACE, please email PLOS at [email protected] . Please note that Supporting Information files do not need this step.
Author response to Decision Letter 0
Collection date 2022.
20 Jul 2022
Reviewer #1:
First of all, we would like to thank for the comments and constructive suggestions that will certainly contribute to the enrichment of this study. In this revised version, we reinforce all the points suggested by the reviewer, especially in the methods and in the discussion part. The changes are highlighted in yellow in the revised manuscript with track changes version.
In the 'Information Sources' chapter, we reinforce the criteria that were applied to choose data sources, based on the Scimago ranking. The search strategy was also completed, reinforcing the filters and terms used for the search. We also combine the search strategy with the eligibility criteria. In the discussion part, we refer to other systematic review studies that address the Clinical Decision Support System (DSS), but that do not relate to the objective of this study. They tend to analyse the application/performance of DSS into specific clinical problems, while we intend to analyse and characterize DSS by their capacities supporting the phases decision making process.
In the Discussion and Conclusion chapters, we rewrote as suggested, presenting a clearer, literature-based structure. We hope that the changes made are as expected.
Reviewer #2:
First of all, we would like to thank for the comments and constructive suggestions that will certainly contribute to the enrichment of this study. In this revised version, we reinforce all the points suggested by the reviewer, especially in the methods part. The changes are highlighted in green in the revised manuscript with track changes version. In the Data Extraction and Management chapter, we describe all the information that was extracted from the reviewed articles. In addition, we highlighted what was expected to be found at each stage of the decision-making process. We included the list of conditions considered for classifying DSS according to these phases (Table 2). We hope that the changes made are as expected.
Submitted filename: Response to Reviewers.docx
Decision Letter 1
28 Jul 2022
Towards Effective Clinical Decision Support Systems: A Systematic Review
PONE-D-20-38355R1
We’re pleased to inform you that your manuscript has been judged scientifically suitable for publication and will be formally accepted for publication once it meets all outstanding technical requirements.
Within one week, you’ll receive an e-mail detailing the required amendments. When these have been addressed, you’ll receive a formal acceptance letter and your manuscript will be scheduled for publication.
An invoice for payment will follow shortly after the formal acceptance. To ensure an efficient process, please log into Editorial Manager at http://www.editorialmanager.com/pone/ , click the 'Update My Information' link at the top of the page, and double check that your user information is up-to-date. If you have any billing related questions, please contact our Author Billing department directly at [email protected] .
If your institution or institutions have a press office, please notify them about your upcoming paper to help maximize its impact. If they’ll be preparing press materials, please inform our press team as soon as possible -- no later than 48 hours after receiving the formal acceptance. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information, please contact [email protected] .
Additional Editor Comments (optional):
Both reviewers recommend acceptance. I agree with their judgement and I recommend acceptance as well.
1. If the authors have adequately addressed your comments raised in a previous round of review and you feel that this manuscript is now acceptable for publication, you may indicate that here to bypass the “Comments to the Author” section, enter your conflict of interest statement in the “Confidential to Editor” section, and submit your "Accept" recommendation.
Reviewer #1: All comments have been addressed
Reviewer #2: All comments have been addressed
2. Is the manuscript technically sound, and do the data support the conclusions?
3. Has the statistical analysis been performed appropriately and rigorously?
Reviewer #2: N/A
4. Have the authors made all data underlying the findings in their manuscript fully available?
5. Is the manuscript presented in an intelligible fashion and written in standard English?
6. Review Comments to the Author
Reviewer #1: Authors have adequately addressed my comments raised in a previous round of review and my feel that this manuscript is now acceptable for publication.
With respect
Five open source online resources are considered. In addition, defined a methodology based on specific criteria: freely accessible articles, written in English, must provide medical support, media must be computer or technological. Two reviewers will analyze the title, abstract and keyword of the articles according to the parameters and will extract the most significant ones. The extracted elements will be read entirely and analyzed. In particular, four main features will be analyzed: the management and technical representations, technological characteristics of the system, the type of system and the integration of the system. Authors express clearly the choice of criteria and the analysis made. In addition, the differences and aspects highlighted by the collection are express.
7. PLOS authors have the option to publish the peer review history of their article ( what does this mean? ). If published, this will include your full peer review and any attached files.
Reviewer #1: No
Acceptance letter
Dear Dr. Hak:
I'm pleased to inform you that your manuscript has been deemed suitable for publication in PLOS ONE. Congratulations! Your manuscript is now with our production department.
If your institution or institutions have a press office, please let them know about your upcoming paper now to help maximize its impact. If they'll be preparing press materials, please inform our press team within the next 48 hours. Your manuscript will remain under strict press embargo until 2 pm Eastern Time on the date of publication. For more information please contact [email protected] .
If we can help with anything else, please email us at [email protected] .
Thank you for submitting your work to PLOS ONE and supporting open access.
PLOS ONE Editorial Office Staff
on behalf of
Dr. Gabriele Oliva
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data availability statement.
- View on publisher site
- PDF (1.1 MB)
- Collections
Similar articles
Cited by other articles, links to ncbi databases.
- Download .nbib .nbib
- Format: AMA APA MLA NLM
Add to Collections
Thank you for visiting nature.com. You are using a browser version with limited support for CSS. To obtain the best experience, we recommend you use a more up to date browser (or turn off compatibility mode in Internet Explorer). In the meantime, to ensure continued support, we are displaying the site without styles and JavaScript.
- View all journals
- My Account Login
- Explore content
- About the journal
- Publish with us
- Sign up for alerts
- Review Article
- Open access
- Published: 06 February 2020
An overview of clinical decision support systems: benefits, risks, and strategies for success
- Reed T. Sutton ORCID: orcid.org/0000-0002-3009-1914 1 ,
- David Pincock 2 ,
- Daniel C. Baumgart 1 ,
- Daniel C. Sadowski 1 ,
- Richard N. Fedorak 1 &
- Karen I. Kroeker 1
npj Digital Medicine volume 3 , Article number: 17 ( 2020 ) Cite this article
390k Accesses
1113 Citations
128 Altmetric
Metrics details
- Drug regulation
- Health services
- Medical imaging
Computerized clinical decision support systems, or CDSS, represent a paradigm shift in healthcare today. CDSS are used to augment clinicians in their complex decision-making processes. Since their first use in the 1980s, CDSS have seen a rapid evolution. They are now commonly administered through electronic medical records and other computerized clinical workflows, which has been facilitated by increasing global adoption of electronic medical records with advanced capabilities. Despite these advances, there remain unknowns regarding the effect CDSS have on the providers who use them, patient outcomes, and costs. There have been numerous published examples in the past decade(s) of CDSS success stories, but notable setbacks have also shown us that CDSS are not without risks. In this paper, we provide a state-of-the-art overview on the use of clinical decision support systems in medicine, including the different types, current use cases with proven efficacy, common pitfalls, and potential harms. We conclude with evidence-based recommendations for minimizing risk in CDSS design, implementation, evaluation, and maintenance.

Similar content being viewed by others
The potential of artificial intelligence to improve patient safety: a scoping review

Why do probabilistic clinical models fail to transport between sites
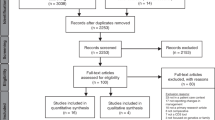
Effect of genetics clinical decision support tools on health-care providers’ decision making: a mixed-methods systematic review
Introduction: what is a clinical decision support system.
A clinical decision support system (CDSS) is intended to improve healthcare delivery by enhancing medical decisions with targeted clinical knowledge, patient information, and other health information. 1 A traditional CDSS is comprised of software designed to be a direct aid to clinical-decision making, in which the characteristics of an individual patient are matched to a computerized clinical knowledge base and patient-specific assessments or recommendations are then presented to the clinician for a decision. 2 CDSSs today are primarily used at the point-of-care, for the clinician to combine their knowledge with information or suggestions provided by the CDSS. Increasingly however, there are CDSS being developed with the capability to leverage data and observations otherwise unobtainable or uninterpretable by humans.
Computer-based CDSSs can be traced to the 1970s. At the time, they had poor system integration, were time intensive and often limited to academic pursuits. 3 , 4 There were also ethical and legal issues raised around the use of computers in medicine, physician autonomy, and who would be at fault when using the recommendation of a system with imperfect ‘explainability’. 5 Presently, CDSS often make use of web-applications or integration with electronic health records (EHR) and computerized provider order entry (CPOE) systems. They can be administered through desktop, tablet, smartphone, but also other devices such as biometric monitoring and wearable health technology. These devices may or may not produce outputs directly on the device or be linked into EHR databases. 6
CDSSs have been classified and subdivided into various categories and types, including intervention timing, and whether they have active or passive delivery. 7 , 8 CDSS are frequently classified as knowledge-based or non-knowledge based. In knowledge-based systems, rules (IF-THEN statements) are created, with the system retrieving data to evaluate the rule, and producing an action or output 7 ; Rules can be made using literature-based, practice-based, or patient-directed evidence. 2 CDSS that are non-knowledge based still require a data source, but the decision leverages artificial intelligence (AI), machine learning (ML), or statistical pattern recognition, rather than being programmed to follow expert medical knowledge. 7 Non-knowledge based CDSS, although a rapidly growing use case for AI in medicine, are rife with challenges including problems understanding the logic that AI uses to produce recommendations (black boxes), and problems with data availability. 9 They have yet to reach widespread implementation. Both types of CDSS have common components with subtle differences, illustrated in Fig. 1 .
They are composed of (1) base: the rules that are programmed into the system (knowledge-based), the algorithm used to model the decision (non-knowledge based), as well as the data available, (2) inference engine: takes the programmed or AI-determined rules, and data structures, and applies them to the patient’s clinical data to generate an output or action, which is presented to the end user (eg. physician) through the (3) communication mechanism: the website, application, or EHR frontend interface, with which the end user interacts with the system 9 .
CDSS have been endorsed by the US Government’s Health and Medicare acts, financially incentivizing CDS implementation into EHRs. 10 In 2013, an estimated 41% of U.S. hospitals with an EHR, also had a CDSS, and in 2017, 40.2% of US hospitals had advanced CDS capability (HIMSS Stage 6). 11 Elsewhere, adoption rates of EMRs have been promising, with approximately 62% of practitioners in Canada in 2013. 12 Canada has had significant endorsement from the government level, as well as Infoway, a not-for-profit corporation. 13 England has also been a world leader in healthcare IT investment, with up to 20 billion euros invested back in 2010. 13 Several countries have also managed to implement national health records, at least for patient-facing data, including Denmark, Estonia, Australia, and others. 14
The scope of functions provided by CDSS is vast, including diagnostics, alarm systems, disease management, prescription (Rx), drug control, and much more. 15 They can manifest as computerized alerts and reminders, computerized guidelines, order sets, patient data reports, documentation templates, and clinical workflow tools. 16 Each CDSS function will be discussed in detail throughout this review, with the potential and realized benefits of these functions, as well as unintended negative consequences, and strategies to avoid harm from CDSS. Methodology used to inform the review is shown in Box 1 .
Box 1. Methods and sources used for this overview
MEDLINE search 1980-January 2018. Key words: CDSS, diagnostic decision support system/DDSS, personal health record/PHR decision support, EHR decision support
Hand searches of the references of retrieved literature
University libraries searching for texts on clinical decision support systems and other keywords mentioned above
Personal and local experience working with healthcare technology and decision support systems
Functions and advantages of CDSS
Patient safety.
Strategies to reduce medication errors commonly make use of CDSS (Table 1 ). Errors involving drug-drug interactions (DDI) are cited as common and preventable, with up to 65% of inpatients being exposed to one or more potentially harmful combinations. 17 CPOE systems are now designed with drug safety software that has safeguards for dosing, duplication of therapies, and DDI checking. 18 The types of alerts generated by these systems are among the most disseminated kind of decision support. 19 However, studies have found a high level of variability between how alerts for DDIs are displayed (e.g., passive or active/disruptive), which are prioritized, 20 , 21 and in the algorithms used to identify DDIs. 18 , 22 Systems often have varying degrees of irrelevant alerts presented, and there is no standard for how best to implement which alerts to providers. The US Office of the National Coordinator for Health Information Technology has developed a list of ‘high-priority’ list of DDIs for CDS, which has reached various levels of endorsement and deployment in CDSS’ of other countries including the U.K., Belgium, and Korea. 20 , 21 , 23
Other systems targeting patient safety include electronic drug dispensing systems (EDDS), and bar-code point-of-care (BPOC) medication administration systems. 24 These are often implemented together to create a ‘closed loop’, where each step of the process (prescribing, transcribing, dispensing, administering) is computerized and occurs within a connected system. At administration, the medication is automatically identified through radio-frequency identification (RFID) or barcodes and crosschecked with patient information and prescriptions. This presents another target for CDSS and the potential benefit is the prevention of medication administration errors occurring at the ‘bedside’ (opposed to further upstream). Adoption is relatively low, partly due to high technology requirements and costs. 25 However; studies show good efficacy for these systems in reducing errors. 26 Mohoney et al. showed that many of these systems can be combined with CPOE and CDSS simultaneously, with reduced prescribing error rates for drug allergy detection, excessive dosing, and incomplete or unclear ordering. 24 As with most CDSS, errors can still be made if providers omit or deliberately work around the technology. 27
CDSS also improve patient safety through reminder systems for other medical events, and not just those that are medication related. Among numerous examples, a CDSS for blood glucose measurement i n the ICU was able to decrease the number of hypoglycemia events. 28 This CDSS automatically prompted nurses to take a glucose measurement according to a local glucose monitoring protocol, which specified how often measurements should be done according to specific patient demographics and previous glucose levels/trends. 28
Overall, CDSS targeting patient safety through CPOE and other systems have generally been successful in reducing prescribing and dosing errors, contraindications through automated warnings, drug-event monitoring and more. 29 Patient safety can be considered a secondary objective (or requirement) of almost all types of CDSS, no matter the primary purpose for their implementation.
Clinical management
Studies have shown CDSS can increase adherence to clinical guidelines. 30 This is significant because traditional clinical guidelines and care pathways have been shown to be difficult to implement in practice with low clinician adherance. 31 , 32 The assumption that practitioners will read, internalize, and implement new guidelines has not held true. 33 However, the rules implicitly encoded in guidelines can be literally encoded into CDSS. Such CDSS can take a variety of forms, from standardized order sets for a targeted case, alerts to a specific protocol for the patients it pertains to, reminders for testing, etc. Furthermore, CDSS can assist with managing patients on research/treatment protocols, 34 tracking and placing orders, follow-up for referrals, as well as ensuring preventative care. 35
CDSS can also alert clinicians to reach out to patients who have not followed management plans, or are due for follow-up, and help identify patients eligible for research based on specific criteria. 36 A CDSS designed and implemented at Cleveland Clinic provides a point-of-care alert to physicians when a patient’s record matches clinical trial criteria. 37 The alert prompts the user to complete a form which establishes eligibility and consent-to-contact, forwards the patient’s chart to the study coordinator, and prints a clinical trial patient information sheet.
Cost containment
CDSS can be cost-effective for health systems, through clinical interventions, 38 decreasing inpatient length-of-stay, CPOE-integrated systems suggesting cheaper medication alternatives, 39 or reducing test duplication. A CPOE-rule was implemented in a pediatric cardiovascular intensive care unit (ICU) that limited the scheduling of blood count, chemistry and coagulation panels to a 24-h interval. 40 This reduced laboratory resource utilization with a projected cost savings of $717,538 per year, without increasing length of stay (LOS), or mortality.
CDSS can notify the user of cheaper alternatives to drugs, or conditions that insurance companies will cover. In Germany, many inpatients are switched to drugs on hospital drug formularies. After finding that 1 in 5 substitutions were incorrect, Heidelberg hospital developed a drug-switch algorithm and integrated it into their existing CPOE system. 41 The CDSS could switch 91.6% of 202 medication consultations automatically, with no errors, increasing safety, reducing workload and reducing cost for providers.
Administrative functions
CDSS provide support for clinical and diagnostic coding, ordering of procedures and tests, and patient triage. Designed algorithms can suggest a refined list of diagnostics codes to aid physicians in selecting the most suitable one(s). A CDSS was conceived to address inaccuracy of ICD-9 emergency department(ED) admission coding (ICD is International Statistical Classification of Diseases, standardized codes used to represent diseases and diagnoses). 42 The tool used an anatomographical interface (visual, interactive representation of the human body) linked to ICD codes to help ED physicians accurately find diagnostic admission codes faster.
CDSS can directly improve quality of clinical documentation. An obstetric CDSS featured an enhanced prompting system, significantly improving documentation of indications for labor induction and estimated fetal weight, compared to control hospital. 43 Documentation accuracy is important because it can directly aid clinical protocols. For example, a CDSS was implemented to ensure patients were properly vaccinated following splenectomy, to combat the increased risk of infections (including pneumococcal, Haemophilus influenzae , meningococcal, etc.) that comes with spleen removal. However, the authors found that 71% of patients with the term ‘splenectomy’ in their EHR did not have it documented on their problem list (which was what triggers the CDSS alert). 44 A supplemental CDSS was then developed to enhance problem list documentation of splenectomy, 45 and improve the utility of the original vaccination CDSS.
Diagnostics support
CDSS for clinical diagnosis are known as diagnostic decision support systems (DDSS). These systems have traditionally provided a computerized ‘consultation’ or filtering step, whereby they might be provided data/user selections, and then output a list of possible or probable diagnoses. 46 Unfortunately, DDSS have not had as much influence as other types of CDSS (yet) for reasons including negative physician perceptions and biases, poor accuracy (often due to gaps in data availability), and poor system integration requiring manual data entry. 47 , 48 The latter is improving with better EHR-integration and standardized vocabulary like Snomed Clinical Terms.
A good example of an effective DDSS is one which was created by Kunhimangalam et al. 49 for diagnosis of peripheral neuropathy using fuzzy logic. Through 24 input fields which include symptoms and diagnostic test outputs, they achieved 93% accuracy compared to experts at identifying motor, sensory, mixed neuropathies, or normal cases. While this has great utility, especially in countries with less access to established clinical experts, there is also a desire for systems that can supplement specialist diagnostics. DXplain is an electronic reference based DDSS that provides probable diagnosis based on clinical manifestations. 50 In a randomized control trial involving 87 family medicine residents, those randomized to use the system showed significantly higher accuracy (84% vs. 74%) on a validated diagnosis test involving 30 clinical cases. 50
Given the known incidence of diagnostic errors, particularly in primary care, 51 there is a lot of hope for CDSS and IT solutions to bring improvements to diagnosis. 52 We are now seeing diagnostic systems being developed with non-knowledge-based techniques like machine learning, which may pave the way for more accurate diagnosis. The Babylon AI powered Triage and Diagnostic System in the U.K. is a good example of the potential, but also of the work that still has to be done before these systems are ready for primetime. 53 , 54
Diagnostics support: imaging
Knowledge-based imaging CDSS are typically used for image ordering, where CDSS can aid radiologists in selecting the most appropriate test to run, providing reminders of best practice guidelines, or alerting contraindications to contrast, for example. 55 An interventional CDS for image ordering at Virginia Mason Medical Center was shown to substantially decrease the utilization rate of lumbar MRI for low back pain, head MRI for headache, and sinus CT for sinusitis. 56 The CDS required a series of questions to be answered by providers prior to image ordering (POC), to verify appropriateness. Importantly, if an image was denied, an alternative was suggested by the system. Another commercialized example is RadWise®, which guides clinicians to the most relevant imaging order by analyzing patient symptoms and matching them with a large database of diagnoses, while also providing appropriate use recommendations at the point of care. 57
There is great interest in non-knowledge based CDS for enhanced imaging and precision radiology (‘radiomics’). 58 , 59 With images accounting for increasing amounts of medical data, but requiring extensive manual interpretation, providers need technologies to aid them in extracting, visualizing, and interpreting. 60 AI technologies are proving capable of providing insights into data beyond what humans can. 61 To do so, these technologies make use of advanced pixel recognition and image classification algorithms, most prominently: deep learning (DL). 62 IBM Watson Health, DeepMind, Google, and other companies are at the forefront, developing products for use in tumor detection, 63 medical imaging interpretation, 64 diabetic retinopathy diagnosis, 65 Alzheimer’s diagnosis through multimodal feature learning, 62 and countless more. IBM Watson’s ‘Eyes of Watson’, has been able to combine image recognition of a brain scan with text recognition of case descriptions to provide comprehensive decision support (or what IBM describes as a ‘cognitive assistant’). 60
Several projects have been able to demonstrate performance that is disputably ‘on par’ with human experts. 65 , 66 , 67 , 68 For example, Google’s team trained a deep convolutional neural network (CNN) to detect diabetic retinopathy (blood vessel damage in the eye) from a dataset of 130,000 retinal images with a very high sensitivity and specificity. 65 The algorithms performance was on par with US board certified ophthalmologists. Another study just recently published by the Stanford group demonstrated a CNN for detecting arrhythmias on electrocardiogram that exceeded the accuracy (F1 and sensitivity with matched specificity) of the average cardiologist on all rhythm classes. 68 With the current rate of progress, some experts controversially speculate that in 15–20 years, the majority of diagnostic imaging interpretation will be done (or at least pre-processed) by computers. 69 For the time being however, we should think of these early systems as an addition or augmentation to a clinician’s available toolset.
Diagnostics support: laboratory and pathology
Another subset of diagnostics where CDSS can be useful is laboratory testing and interpretation. Alerts and reminders for abnormal lab results are simple and ubiquitous in EHR systems. CDSS can also extend the utility of lab-based tests for the purpose of avoiding riskier or more invasive diagnostics. In Hepatitis B and C testing, liver biopsies are considered the gold standard for diagnosis, while non-invasive lab tests are not accurate enough to be accepted. However; AI models are being developed that combine multiple tests (serum markers, imaging, and gene tests) to produce much greater accuracy. 70 There is also application for CDSS as an interpretation tool where a test’s reference ranges are highly personalized, for example age, sex, or disease subtypes. 71
Pathology reports are crucial as decision points for many other medical specialties. Some CDSS can be used for automated tumor grading. This was done for urinary bladder tumor grading and estimating recurrence, with up to 93% accuracy. 72 The same has been done for brain tumor classification and grading. 73 There are many other examples including computerized ECG analysis, automated arterial blood gas interpretation, protein electrophoresis reports, and CDSS for blood cell counting. 46
Patient-facing decision support
With the advent of the ‘Personal Health Record’ (PHR), we are seeing CDS functionality integrated, similar to EHRs, with the patient as the end user or ‘manager’ of the data. This is a great step towards patient-focused care, and CDS-supported PHRs are the ideal tool to implement shared decision-making between patient and provider, specifically because CDSS can remove a ‘lack of information’ as a barrier to a patient’s participation in their own care. 74 PHRs are frequently designed as an extension of commercial EHR software, or as standalone web-based or mobile-based applications. 75 When connected to EHRs, PHRs can have a two way relationship, whereby information entered directly by the patient can be available to their providers, and also information in the EHR can be transmitted to the PHR for patients to view. 76
One of the earliest PHRs, the “Patient Gateway”, was simply a dashboard for patients to view medications and labs, and communicate with their physicians. 77 This has expanded and some systems now allow patients to modify their own record of care, effecting the EHR data as well. 78 Another example is Vanderbilt University’s MyHealthAtVanderbilt, a PHR fully integrated into the institutional EHR. In addition to disease-targeted delivery of patient educational materials, they incorporated a Flu Tool for patients with flu-like symptoms to decide the level of care they need and then help them seek treatment. 79 Symptom tracking is a useful and common feature of PHRs, but the variety of collected data is virtually limitless, from allergies to insurance coverage to prescription and medication information. 80 Furthermore, PHRs and other patient monitoring applications can be designed to collect information from health devices and other wearables, to create actionable insights for providers. An excellent example exists in diabetes care. Many systems are already in use, 81 but one in particular pioneered by the Stanford School of Medicine uses a wearable glucose monitor which transmits data to an Apple device (HealthKit). 82 Apple has made HealthKit interoperable with the Epic EHR and Epic PHR, “MyChart”. This successfully allows providers to monitor glucose trends in their patients in between visits, and contact them through MyChart for follow up or urgent recommendations. The pilot study demonstrated improved provider workflow, communication with patients, and ultimately quality of care. 82 Various other medical fields are deploying similar systems for monitoring that combines PHR/EHR, wearable technologies, and CDSS, including but not limited to heart failure (cardiology), hypertension, sleep apnea, palliative/elder care, and more.
It is worth noting that as PHRs have become more advanced with CDSS capabilities, there has also been increasing emphasis on the design of these systems to serve shared decision making between patient and provider, and to be interactive tools to make patients more knowledgeable/involved in their own care. PHRs that only serve as a repository for health information are now seen as missing the mark, particularly by patients themselves. 75
Pitfalls of CDSS
Fragmented workflows.
CDSS can disrupt clinician workflow, especially in the case of stand-alone systems. Many early CDSS were designed as systems that required the provider to document or source information outside their typical workspace. CDSS also disrupt workflow if designed without human information processing and behaviors in mind. In response, CDSS have been designed using the ‘think-aloud’ method to model practitioners’ workflow and create a system with better usability. 83
Disrupted workflow can lead to increased cognitive effort, more time required to complete tasks, and less time face-to-face with patients. Even when CDSS are well integrated within existing information systems, there can be disconnect between face-to-face interactions and interaction with a computer workstation. Studies have found that practitioners with more experiential knowledge are less likely to use, and more likely to override CDSS. 84
Alert fatigue and inappropriate alerts
Studies have found up to 95% of CDSS alerts are inconsequential, and often times physicians disagree with or distrust alerts. 85 Other times they just do not read them. If physicians are presented with excessive/unimportant alerts, they can suffer from alert fatigue. 86
Disruptive alerts should be limited to more life-threatening or consequential contraindications, such as serious allergies. However; even allergy alerts can be incorrect, and clinicians will often verify themselves, especially if the source is another site/hospital/practitioner. 85 , 87 Medication alerts can also be specialty specific, but irrelevant when taken out of context. For example, an alert against using broad-spectrum antibiotics such as vancomycin may be inappropriate in ICU. 85 An alert against duplicate medications may be inappropriate in inflammatory bowel disease clinics, where the same class of drug can be applied through different administration routes for increased effect.
Impact on user skill
Prior to CPOE and CDSS, healthcare providers, pharmacists, and nurses were relied upon exclusively to double-check orders. CDSS can create the impression that verifying the accuracy of an order is unnecessary or automatic. 85 This is an important myth to dispel.
It is also important to consider the potential long-term effect of a CDSS on users. Over time a CDSS can exert a training effect, so that the CDSS itself may no longer be required. Coined the “carry-over effect”, it is most likely with CDSS that are educational in nature. 88 Conversely, providers may develop too much reliance or trust on a CDSS for a specific task. 89 This could be compared to using a calculator for mathematical operations over a long period of time, and then having poorer mental math skills. It is potentially problematic as the user has less independence and will be less equipped for that task should they switch to an environment without the CDSS.
CDSS may be dependent on computer literacy
Lack of technological proficiency can be hindering when engaging with a CDSS. 90 , 91 This can vary by the design details of the CDSS, but some have been found to be overly complex, relying too much on user skill. 90 , 92 Systems should aim to stay as close to the core functionality of the pre-existing system as possible. Regardless, all new systems have a learning period, and so baseline evaluations of users’ technological competence may be appropriate. Further training can then be provided to facilitate full use of CDSS capabilities, 93 or more explicit guidance incorporated into the CDSS’ recommendations themselves. 94 This information could be implemented as info buttons to be non-disruptive. 95
System and content maintenance
Maintenance of CDSS is an important but often neglected part of the CDSS life-cycle. This includes technical maintenance of systems, applications and databases that power the CDSS. Another challenge is the maintenance of knowledge-base and its rules, which must keep apace with the fast-changing nature of medical practice and clinical guidelines. Even the most advanced healthcare institutions report difficulty keeping their systems up to date as knowledge inevitably changes. 85 Order sets and the algorithmic rules behind the CDSS have been identified as particularly difficult. 85
Operational impact of poor data quality and incorrect content
EHRs and CDSSs rely on data from external, dynamic systems and this can create novel deficiencies. As an example, some CDSS modules might encourage ordering even when the hospital lacks adequate supplies. In a study by Ash et al. 85 , a number of experts indicated that at their hospital, Hemoccult tests or pneumococcal vaccine inventories run out quickly, but this is not communicated to the CDSS.
Medication and problem lists can be problematic, if not updated or used appropriately. At one site, the medication list might be a list of dispensations, which means patients may or may not be taking them(and thus must still be asked in person). 85 Other medication lists are generated from CPOE orders only, thus still requiring manual confirmation that patients are taking the medication. Systems that make it easy to distinguish these are ideal. It is also a major area where PHRs could create a solution, by collecting medication adherence data directly from patients.
In poorly designed systems, users may develop workarounds that compromise data, such as entering generic or incorrect data. 85 The knowledge base of CDSS is dependent on a centralized, large clinical data repository. Quality of data can affect quality of decision support. If data collection or input into the system is unstandardized, the data is effectively corrupted. You may design a system for use at the point-of-care, but when applied to real world environments and data, will not be utilized properly. The importance of using informational standards such as ICD, SNOMED, and others, cannot be understated.
Lack of transportability and interoperability
Despite ongoing development for the better part of three decades, CDSS (and even EHRs in general) suffer from interoperability issues. Many CDSS exist as cumbersome stand-alone systems, or exist in a system that cannot communicate effectively with other systems.
What makes transportability so difficult to achieve? Beyond programming complexities that can make integration difficult, the diversity of clinical data sources is a challenge. 96 There is a reluctance or perceived risk associated with transporting sensitive patient information. Positively, interoperability standards are continuously being developed and improved, such as Health Level 7 (HL7) and Fast Healthcare Interoperability Resources (FHIR). These are already being utilized in commercial EHR vendors. 97 Several government agencies, medical organizations and informatics bodies are actively supporting and some even mandating the use of these interoperability standards in health systems. 98 , 99 , 100
The cloud also offers a potential solution to interoperability (and other EHR ailments such as data sync, software updating, etc. 101 ). Cloud EHRs have open architecture, newer standards, and more flexible connectivity to other systems. 102 It is also a common misconception that data stored on a cloud is more vulnerable. This is not necessarily true. Web-based EHRs are required to store data in high-level storage centers with advanced encryption and other safeguards. They must comply with national data security standards including the Health Insurance Portability and Accountability Act (HIPAA) in the USA, Personal Information Protection and Electronic Documents Act (PIPEDA) in Canada, or the Data Protection Directive and General Data Protection Regulation (GDPR) in Europe, to name a few. 103 They can be just as safe (or just as vulnerable) as traditional, server-based architecture. 103 In fact, there are often fewer people who have access to unencrypted data in cloud storage centers vs. server-based records. 103
Financial challenges
Up to 74% of those with a CDSS said that financial viability remains a struggle. 104 Outset costs to set up and integrate new systems can be substantial. Ongoing costs can continue to be an issue indefinitely as new staff need to be trained to use the system, and system updates are required to keep pace with current knowledge.
Results from cost analyses of CDSS implementations are mixed, controversial, and sparse. 105 , 106 , 107 , 108 Whether an intervention is cost-effective depends on a wide range of factors, including those specific to the environment, both political and technological. 105 Cost benefit assessment in itself can be limited, with challenges such as a lack of standardized metrics. 107 This is an emerging research area and much work needs to be done to advance our understanding of the financial effects of CDSS.
CDSS have been shown to augment healthcare providers in a variety of decisions and patient care tasks, and today they actively and ubiquitously support delivery of quality care. Some applications of CDSS have more evidence behind them, especially those based on CPOE. Support for CDSS continues to mount in the age of the electronic medical record, and there are still more advances to be made including interoperability, speed and ease of deployment, and affordability. At the same time, we must stay vigilant for potential downfalls of CDSS, which range from simply not working and wasting resources, to fatiguing providers and compromising quality of patient care. Extra precautions and conscientious design must be taken when building, implementing, and maintaining CDSS. A portion of these considerations were covered in this review, but further review will be required in practice, especially as CDSS continue to evolve in complexity through advances in AI, interoperability, and new sources of data.
Osheroff, J. et al. Improving Outcomes with Clinical Decision Support: An Implementer’s Guide . (HIMSS Publishing, 2012).
Sim, I. et al. Clinical decision support systems for the practice of evidence-based medicine. J. Am. Med Inf. Assoc. Jamia. 8 , 527–534 (2001).
Article CAS Google Scholar
De Dombal, F. Computers, diagnoses and patients with acute abdominal pain. Arch. Emerg. Med . 9 , 267–270 (1992).
Shortliffe, E. H. & Buchanan, B. G. A model of inexact resoning in medicine. Math. Biosci. 379 , 233–262 (1975).
Google Scholar
Middleton, B., Sittig, D. F. & Wright, A. Clinical decision support: a 25 year retrospective and a 25 year vision. Yearb. Med. Inform. 25 (S 01), S103–S116 (2016).
Article Google Scholar
Dias, D. Wearable health devices—vital sign monitoring, systems and technologies. https://doi.org/10.3390/s18082414 (2018).
Berner, E. S. (Ed.). Clinical Decision Support Systems (Springer, New York, NY, 2007).
Osheroff, J., Pifer, E., Teigh, J., Sittig, D. & Jenders, R. Improving Outcomes with Clinical Decision Support: An Implementer’s Guide. (HIMS, 2005).
Deo, R. C. Machine learning in medicine. Circulation 132 , 1920–1930 (2015).
Article PubMed PubMed Central Google Scholar
HITECH Act Enforcement Interim Final Rule. https://www.hhs.gov/hipaa/for-professionals/special-topics/HITECH-act-enforcement-interim-final-rule/index.html .
Electronic medical record adoption model requirements. https://www.himssanalytics.org/emram , Accessed 25 Aug 2019 (2017).
Chang, F. & Gupta, N. Progress in electronic medical record adoption in Canada Recherche Les progrès dans l’ adoption du dossier médical électronique au Canada. Canadian Family Physician. 61 , 1076–1084 (2015).
Healthcare Information and Management Systems Society (HIMSS). Electronic Health Records: A Global Perspective, 2nd edn. https://s3.amazonaws.com/rdcms-himss/files/production/public/HIMSSorg/Content/files/Globalpt1-edited%20final.pdf (2010).
Nøhr, C. et al. Nationwide citizen access to their health data: analysing and comparing experiences in Denmark, Estonia and Australia. 1–11. https://doi.org/10.1186/s12913-017-2482-y (2017).
Omididan, Z. & Hadianfar, A. The role of clinical decision support systems in healthcare (1980-2010): a systematic review study. Jentashapir Sci.-Res Q. 2 , 125–134 (2011).
Kabane, S. M. Healthcare and the Effect of Technology: Developments, Challenges and Advancements: Developments, Challenges and Advancements. Medical Information Science Reference (2010).
Vonbach, P., Dubied, A., Krähenbühl, S. & Beer, J. H. Prevalence of drug-drug interactions at hospital entry and during hospital stay of patients in internal medicine. Eur. J. Intern. Med. 19 , 413–420 (2008).
Article PubMed Google Scholar
Helmons, P. J., Suijkerbuijk, B. O., Nannan Panday, P. V. & Kosterink, J. G. W. Drug-drug interaction checking assisted by clinical decision support: a return on investment analysis. J. Am. Med. Inf. Assoc. Jamia. 22 , 764–772 (2015).
Koutkias, V. & Bouaud, J. Contributions from the 2017 Literature on Clinical Decision Support. Yearb. Med. Inf. 27 , 122–128 (2018).
Phansalkar, S. et al. High-priority drug – drug interactions for use in electronic health records. J. Am. Med. Inform. Assoc . 19 , 735–743 (2012).
Cornu, P., Phansalkar, S., Seger, D. L., Cho, I. & Pontefract, S. International Journal of Medical Informatics High-priority and low-priority drug – drug interactions in di ff erent international electronic health record systems: a comparative study. Int. J. Med. Inf. 111 , 165–171 (2018).
McEvoy, D. S. et al. Variation in high-priority drug-drug interaction alerts across institutions and electronic health records. J. Am. Med. Inf. Assoc. 24 , 331–338 (2017).
Cho, I., Lee, J., Choi, J., Hwang, H. & Bates, D. W. National rules for drug-drug interactions: are they appropriate for tertiary hospitals?. J. Korean Med. Sci. 31 , 1887–1896 (2016).
Mahoney, C. D., Berard-Collins, C. M., Coleman, R., Amaral, J. F. & Cotter, C. M. Effects of an integrated clinical information system on medication safety in a multi-hospital setting. Am. J. Health Syst. Pharm. 64 , 1969–1977 (2007).
Peris-Lopez, P., Orfila, A., Mitrokotsa, A. & van der Lubbe, J. C. A. A comprehensive RFID solution to enhance inpatient medication safety. Int. J. Med. Inf. 80 , 13–24 (2011).
Levtzion-korach, O. et al. Effect of bar-code technology on the safety of medication administration. N. Engl. J. Med. 362 , 1698–1707 (2010).
van der Veen, W. et al. Association between workarounds and medication administration errors in bar-code-assisted medication administration in hospitals. J. Am. Med. Inf. Assoc. 25 , 385–392 (2018).
Eslami, S. et al. Effects of two different levels of computerized decision support on blood glucose regulation in critically ill patients. Int J. Med. Inf. 81 , 53–60 (2012).
Jia, P., Zhang, L., Chen, J., Zhao, P. & Zhang, M. The effects of clinical decision support systems on medication safety: an overview. PLoS ONE 11 , 1–17 (2016).
Kwok, R., Dinh, M., Dinh, D. & Chu, M. Improving adherence to asthma clinical guidelines and discharge documentation from emergency departments: Implementation of a dynamic and integrated electronic decision support system. Emerg. Med. Australas. 21 , 31–37 (2009).
PubMed Google Scholar
Davis, D. A. & Taylor-Vaisey, A. Translating guidelines into practice: a systematic review of theoretic concepts, practical experience and research evidence in the adoption of clinical practice guidelines. Can. Med. Assoc. J. 157 , 408–416 (1997).
CAS Google Scholar
Michael, C., Rand, C. S., Powe, N. R., Wu, A. W. & Wilson, M. H. Why don’ t physicians follow clinical practice guidelines? a framework for improvement. Jama,. 282 , 1458–1465 (1999).
Shortliffe, T. Medical thinking: what should we do? http://www.openclinical.org/medicalThinking2006Summary2.html (2006).
Lipton, J. A. et al. Impact of an alerting clinical decision support system for glucose control on protocol compliance and glycemic control in the intensive cardiac care unit. Diabetes Technol. Ther. 13 , 343–349 (2011).
Salem, H. et al. A multicentre integration of a computer led follow up in surgical oncology is valid and safe. BJU Int . https://doi.org/10.1111/bju.14157 (2018).
Health Information Technology Foundations Module 28: Clinical Decision Support Basics. Carnegie Mellon University Open Learning Initiative. https://oli.cmu.edu/jcourse/workbook/activity/page?context=e6f7c0b180020ca600c0f4e5957d6f8c .
Embi, P. J., Jain, A., Clark, J. & Harris, C. M. Development of an electronic health record-based Clinical Trial Alert system to enhance recruitment at the point of care. AMIA Annu. Symp. Proc. 2005 , 231–235 (2005).
Calloway, S., Akilo, H. & Bierman, K. Impact of a clinical decision support system on pharmacy clinical interventions, documentation efforts, and costs. Hosp. Pharm. 48 , 744–752 (2013).
McMullin, S. T. et al. Impact of an evidence-based computerized decision support system on primary care prescription costs. Ann. Fam. Med. 2 , 494–498 (2004).
Algaze, C. A. et al. Use of a checklist and clinical decision support tool reduces laboratory use and improves cost. Pediatrics 137 , e20143019 (2016).
Pruszydlo, M. G., Walk-Fritz, S. U., Hoppe-Tichy, T., Kaltschmidt, J. & Haefeli, W. E. Development and evaluation of a computerised clinical decision support system for switching drugs at the interface between primary and tertiary care. BMC Med. Inf. Decis. Mak. 12 , 1 (2012).
Bell, C. M., Jalali, A. & Mensah, E. A decision support tool for using an ICD-10 anatomographer to address admission coding inaccuracies: a commentary. Online J. Public Health Inform. 5 , 222 (2013).
Haberman, S. et al. Effect of clinical-decision support on documentation compliance in an electronic medical record. Obstet. Gynecol. 114 , 311–317 (2009).
Turchin, A., Shubina, M. & Gandhi, T. NLP for patient safety: splenectomy and pneumovax. In Proc. AMIA 2010 Annual Symposium (2010).
McEvoy, D., Gandhi, T. K., Turchin, A. & Wright, A. Enhancing problem list documentation in electronic health records using two methods: the example of prior splenectomy. BMJ Qual Saf . https://doi.org/10.1136/bmjqs-2017-006707 (2017).
Berner E. Clinical Decision Support Systems: Theory and Practice 3rd edn. https://doi.org/10.1007/978-0-387-38319-4 (2016).
Berner, E. S. Diagnostic decision support systems: why aren’t they used more and what can we do about it? AMIA Annu. Symp. Proc . 2006 , 1167–1168 (2006).
Segal, M. M. et al. Experience with integrating diagnostic decision support software with electronic health records: benefits versus risks of information sharing. EGEMs Gener. Evid. Methods Improv. Patient Outcomes 5 , 23 (2017).
Kunhimangalam, R., Ovallath, S. & Joseph, P. K. A clinical decision support system with an integrated EMR for diagnosis of peripheral neuropathy. J. Med. Syst. 38 , 38 (2014).
Martinez-Franco, A. I. et al. Diagnostic accuracy in Family Medicine residents using a clinical decision support system (DXplain): a randomized-controlled trial. Diagn. Berl. Ger. 5 , 71–76 (2018).
Singh, H., Schiff, G. D., Graber, M. L., Onakpoya, I. & Thompson, M. J. The global burden of diagnostic errors in primary care. BMJ Qual. Saf. 26 , 484–494 (2017).
Singh, H., Meyer, A. N. D. & Thomas, E. J. The frequency of diagnostic errors in outpatient care: Estimations from three large observational studies involving US adult populations. BMJ Qual. Saf. 23 , 727–731 (2014).
Razzaki, S. et al. A comparative study of artificial intelligence and human doctors for the purpose of triage and diagnosis. arXiv preprint arXiv:1806.10698 (2018).
Fraser, H., Coiera, E. & Wong, D. Safety of patient-facing digital symptom checkers. Lancet 392 , 2263–2264 (2018).
Georgiou, A., Prgomet, M., Markewycz, A., Adams, E. & Westbrook, J. I. The impact of computerized provider order entry systems on medical-imaging services: a systematic review. J. Am. Med. Inf. Assoc. 18 , 335–340 (2011).
Blackmore, C. C., Mecklenburg, R. S. & Kaplan, G. S. Effectiveness of clinical decision support in controlling inappropriate imaging. JACR 8 , 19–25 (2019).
DSS Inc. Radiology Decision Support (RadWise®). https://www.dssinc.com/products/integrated-clinical-products/radwise-radiology-decision-support/ .
Giardino, A. et al. Role of imaging in the era of precision medicine. Acad. Radiol. 24 , 639–649 (2017).
Oakden-rayner, L. et al. Precision Radiology: predicting longevity using feature engineering and deep learning methods in a radiomics framework. Sci. Rep . https://doi.org/10.1038/s41598-017-01931-w (2017).
From Invisible to Visible: IBM Demos AI to Radiologists. https://www-03.ibm.com/press/us/en/pressrelease/51146.ws . Accessed Aug 2019 (2016).
Greenspan, H., Ginneken van, B. & Summers, R. M. Guest editorial deep learning in medical imaging: overview and future promise of an exciting new technique. IEEE Trans. Med. Imaging 35 , 1153–1159 (2016).
Suzuki, K. & Chen, Y. Artificial intelligence in decision support systems for diagnosis in medical imaging. https://doi.org/10.1007/978-3-319-68843-5 (2018).
IBM Watson Health - IBM Watson for Oncology. https://www.ibm.com/watson/health/oncology-and-genomics/oncology/ . Accessed 25 Aug 2019 (2018).
Lunit Inc. https://lunit.io/en/ . Accessed Aug 2019 (2018).
Gulshan, V. et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA - J. Am. Med. Assoc. 316 , 2402–2410 (2016).
Esteva, A. et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 542 , 115 (2017).
Article CAS PubMed Google Scholar
Rajpurkar, P. et al. Chexnet: Radiologist-level pneumonia detection on chest x-rays with deep learning. arXiv preprint ar Xiv:1711.05225 (2017).
Hannun, A. Y. et al. FOCUS | Letters FOCUS | Letters Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network letters | FOCUS letters | FOCUS. Nat. Med . https://doi.org/10.1038/s41591-018-0268-3 (2019).
Erickson, B. J. Machine Intelligence in Medical Imaging (Society for Imaging Informatics, SIIM, 2016).
Keltch, B., Lin, Y. & Bayrak, C. Comparison of AI techniques for prediction of liver fibrosis in hepatitis patients patient facing systems. J. Med. Syst . https://doi.org/10.1007/s10916-014-0060-y (2014).
Mørkrid, L. et al. Continuous age- and sex-adjusted reference intervals of urinary markers for cerebral creatine deficiency syndromes: a novel approach to the definition of reference intervals. Clin. Chem. 61 , 760–768 (2015).
Article PubMed CAS Google Scholar
Spyridonos, P., Cavouras, D., Ravazoula, P. & Nikiforidis, G. A computer-based diagnostic and prognostic system for assessing urinary bladder tumour grade and predicting cancer recurrence. Med. Inf. Internet Med. 27 , 111–122 (2002).
Tsolaki, E. et al. Fast spectroscopic multiple analysis (FASMA) for brain tumor classification: a clinical decision support system utilizing multi-parametric 3T MR data. Int. J. Comput. Assist Radio. Surg. 10 , 1149–1166 (2015).
Davis, S., Roudsari, A., Raworth, R., Courtney, K. L. & Mackay, L. Shared decision-making using personal health record technology: a scoping review at the crossroads. J. Am. Med. Inf. Assoc. 24 , 857–866 (2017).
Fuji, K. T. et al. Standalone personal health records in the United States: meeting patient desires. Health Technol. 2 , 197–205 (2012).
Tang, P. C., Ash, J. S., Bates, D. W., Overhage, J. M. & Sands, D. Z. Personal health records: definitions, benefits, and strategies for overcoming barriers to adoption. J. Am. Med. Inf. Assoc. 13 , 121–126 (2006).
Wald, J. S. et al. A patient-controlled journal for an electronic medical record: issues and challenges. Stud. Health Technol. Inform. 107 (Pt 2), 1166–1170 (2004).
Hanauer, D. A., Preib, R., Zheng, K. & Choi, S. W. Patient-initiated electronic health record amendment requests. J. Am. Med. Inf. Assoc. 21 , 992–1000 (2014).
Rosenbloom, S. T. et al. Triaging patients at risk of influenza using a patient portal. J. Am. Med. Inf. Assoc. 19 , 549–554 (2012).
Roehrs, A., Da Costa, C. A., Da Rosa Righi, R. & De Oliveira, K. S. F. Personal health records: A systematic literature review. J. Med. Internet Res . https://doi.org/10.2196/jmir.5876 (2017).
Benhamou, P. Y. Improving diabetes management with electronic health records and patients’ health records. Diabetes Metab. 37 (Suppl. 4), S53–S56 (2011).
Kumar, R. B., Goren, N. D., Stark, D. E., Wall, D. P. & Longhurst, C. A. Automated integration of continuous glucose monitor data in the electronic health record using consumer technology. J. Am. Med. Inf. Assoc. 23 , 532–537 (2016).
Kilsdonk, E., Peute, L. W., Riezebos, R. J., Kremer, L. C. & Jaspers, M. W. M. Uncovering healthcare practitioners’ information processing using the think-aloud method: From paper-based guideline to clinical decision support system. Int. J. Med. Inf. 86 , 10–19 (2016).
Dowding, D. et al. Nurses’ use of computerised clinical decision support systems: A case site analysis. J. Clin. Nurs. 18 , 1159–1167 (2009).
Ash, J. S., Sittig, D. F., Campbell, E. M., Guappone, K. P. & Dykstra, R. H. Some unintended consequences of clinical decision support systems. AMIA Annu Symp. Proc. AMIA Symp. AMIA Symp. 2007 , 26–30 (2007).
Khalifa, M. & Zabani, I. Improving utilization of clinical decision support systems by reducing alert fatigue: Strategies and recommendations. Stud. Health Technol. Inform. 226 , 51–54 (2016).
Van Laere, S. et al. Clinical decision support systems for drug allergy checking: systematic review. https://doi.org/10.2196/jmir.8206 (2018).
Wyatt, J. & Spiegelhalter, D. Field trials of medical decision-aids: potential problems and solutions. American Medical Informatics Association. 3–7. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2247484/ (1991).
Goddard, K., Roudsari, A. & Wyatt, J. Automation bias - A hidden issue for clinical decision support system use. Stud. Health Technol. Inform. 164 , 17–22 (2011).
Devaraj, S., Sharma, S. K., Fausto, D. J., Viernes, S. & Kharrazi, H. Barriers and facilitators to clinical decision support systems adoption: a systematic review. J. Bus Adm. Res . https://doi.org/10.5430/jbar.v3n2p36 (2014).
Leslie, S. J. et al. Clinical decision support software for management of chronic heart failure: Development and evaluation. Comput Biol. Med. 36 , 495–506 (2006).
Murray, E. et al. Why is it difficult to implement e-health initiatives? A qualitative study. Implement Sci. 6 , 6 (2011).
Lai, F., Macmillan, J., Daudelin, D. H. & Kent, D. M. The potential of training to increase acceptance and use of computerized decision support systems for medical diagnosis. Hum. Factors J. Hum. Factors Erg. Soc. 48 , 95–108 (2006).
Ojeleye, L. Ensuring effective computerised clinical decision support. Prescriber 27 , 54–56 (2016).
Cook, D. A., Teixeira, M. T., Heale, B. S. E., Cimino, J. J. & Del Fiol, G. Context-sensitive decision support (infobuttons) in electronic health records: a systematic review. J. Am. Med Inf. Assoc. 24 , 460–468 (2017).
Sujansky, W. Heterogeneous database integration in biomedicine. J. Biomed. Inform. 34 , 285–298 (2001).
Index - FHIR v3.0.1. (2018). https://www.hl7.org/fhir/index.html . Accessed July 2019.
Katehakis, D. G. Towards the Development of a National eHealth Interoperability Framework to Address Public Health Challenges in Greece. Proceedings of the First International Workshop on Semantic Web Technologies for Health Data Management, SWH@ISWC. 2164 , 1–9 (2018).
EHRIntelligence. 5 Ways States Mandate Health Information Exchange. https://ehrintelligence.com/news/5-ways-states-mandate-health-information-exchange . Accessed Aug 2019 (2015).
European Commission Report. Commission Recommendation on a European Electronic Health Record Exchange Format (C(2019)800) of 6 February 2019. https://ec.europa.eu/digital-single-market/en/news/recommendation-european-electronic-health-record-exchange-form (2019).
Bresnick, J. HealthITAnalytics. Interoperability, Low Costs Make Cloud-Based EHRs a Favorite. https://healthitanalytics.com/news/interoperability-low-costs-make-cloud-based-ehrs-a-favorite . Accessed July 2019 (2015).
Fernández-Cardeñosa, G., De La Torre-Díez, I., López-Coronado, M. & Rodrigues, J. J. P. C. Analysis of cloud-based solutions on EHRs systems in different scenarios. J. Med. Syst. 36 , 3777–3782 (2012).
Rodrigues, J. J. P. C., de la Torre, I., Fernández, G. & López-Coronado, M. Analysis of the security and privacy requirements of cloud-based electronic health records systems. J. Med. Internet Res . 15 , e186 (2013).
Kabachinski, J. A look at clinical decision support systems. Biomed. Instrum. Technol. 47 , 432–434 (2013).
O’Reilly, D., Tarride, J.-E., Goeree, R., Lokker, C. & McKibbon, K. A. The economics of health information technology in medication management: a systematic review of economic evaluations. J. Am. Med. Inf. Assoc. 19 , 423–438 (2012).
Bright, T. J. et al. Effect of clinical decision-support systems: a systematic review. Ann. Intern Med. 157 , 29–43 (2012).
Jacob, V. et al. Cost and economic benefit of clinical decision support systems (CDSS) for cardiovascular disease prevention: a community guide systematic review. J. Am. Med. Inf. Assoc. Jamia. 24 , 669–676 (2017).
Main, C. et al. Computerised decision support systems in order communication for diagnostic, screening or monitoring test ordering: systematic reviews of the effects and cost-effectiveness of systems. Health Technol. Assess. 14 , 1–227 (2010).
CAS PubMed Google Scholar
Scheepers-Hoeks, A. M., Grouls, R. J., Neef, C. & Korsten, H. H. Strategy for implementation and first results of advanced clinical decision support in hospital pharmacy practice. Stud. Health Technol. Inform . 148 , 142–148 (2009).
Edlin, R., McCabe, C., Hulme, C., Hall, P. & Wright, J. Cost effectiveness modelling for health technology assessment. https://doi.org/10.1007/978-3-319-15744-3 (2015).
Vermeulen, K. M. et al. Cost-effectiveness of an electronic medication ordering system (CPOE/CDSS) in hospitalized patients. Int J. Med, Inf. 83 , 572–580 (2014).
Okumura, L. M., Veroneze, I., Burgardt, C. I. & Fragoso, M. F. Effects of a computerized provider order entry and a clinical decision support system to improve cefazolin use in surgical prophylaxis: a cost saving analysis. Pharm. Pract. 14 , 1–7 (2016).
Osheroff, J. A. et al. A roadmap for national action on clinical decision support. J. Am. Med. Inf. Assoc. 14 , 141–145 (2007).
Greenes, R. A. Clinical Decision Support 2nd edn. The Road to Broad Adoption https://doi.org/10.1016/B978-0-12-398476-0.00035-X (2014).
Bonney, W. Impacts and risks of adopting clinical decision support systems. In: Efficient Decision Support Systems - Practice and Challenges in Biomedical Related Domain. IntechOpen https://doi.org/10.5772/711 (2011).
Bates, D. W. et al. Ten commandments for effective clinical decision support: making the practice of evidence-based medicine a reality. J. Am. Med. Inf. Assoc. 10 , 523–530 (2003).
Kawamoto, K. Improving clinical practice using clinical decision support systems: a systematic review of trials to identify features critical to success. Bmj 330 , 765–0 (2005).
Health Level Seven International - Homepage. http://www.hl7.org/ . Accessed 29 Aug 2019 (2018).
IHTSDO. History of SNOMED CT. Ihtsdo. http://www.ihtsdo.org/snomed-ct/what-is-snomed-ct/history-of-snomed-ct (2015).
Marco-Ruiz, L. & Bellika, J. G. Semantic Interoperability in Clinical Decision Support Systems: A Systematic Review. Stud. Health Technol. Inf. 216 , 958 (2015).
Angraal, S., Krumholz, H. M. & Schulz, W. L. Blockchain technology: applications in health care. Circ. Cardiovasc. Qual. Outcomes https://doi.org/10.1161/CIRCOUTCOMES.117.003800 (2017).
Ivan, D. Moving toward a blockchain-based method for the secure storage of patient records. ONC/NIST Use of Blockchain for Healthcare and Research Workshop . Gaithersburg, Maryland, United States: ONC/NIST (2016).
Eichner, J. & Das, M. Challenges and Barriers to Clinical Decision Support (CDS) Design and Implementation Experienced in the Agency for Healthcare Research and Quality CDS Demonstrations. Agency Healthc Res. Qual. Website . 29. https://healthit.ahrq.gov/sites/default/files/docs/page/CDS_challenges_and_barriers.pdf (2010).
Khalifa, M. Clinical decision support: strategies for success. Procedia Comput. Sci. 37 , 422–427 (2014).
Sittig, D. F. Electronic Health Records: Challenges in Design and Implementation . (CRC Press, 2014).
Harper, B. D. & Norman, K. L. Improving User Satisfaction: The Questionnaire for User Interaction Satisfaction Version 5.5. Proceedings of the 1st Annual Mid-Atlantic Human Factors Conference, (pp. 224–228), Virginia Beach, VA. (1993).
Lewis, J. R. The system usability scale: past, present, and future. Int J. Hum.-Comput. Interact. 34 , 577–590 (2018).
Lewis, J. R. IBM computer usability satisfaction questionnaires: psychometric evaluation and instructions for use. Int J. Hum.-Comput. Interact. 7 , 57–78 (1995).
Lewis, J. R. Measuring perceived usability: the CSUQ, SUS, and UMUX. Int J. Hum.-Comput Interact. 34 , 1148–1156 (2018).
Download references
Acknowledgements
The author’s (R.T.S.) work was supported by a Canada Institute of Health (CIHR) Research Graduate Scholarship (CGS-M). Thank you to Nathan Stern for discussion and initial review of the manuscript.
Author information
Authors and affiliations.
Department of Medicine, Division of Gastroenterology, University of Alberta, Edmonton, Canada
Reed T. Sutton, Daniel C. Baumgart, Daniel C. Sadowski, Richard N. Fedorak & Karen I. Kroeker
Chief Medical Information Office, Alberta Health Services, Edmonton, Canada
David Pincock
You can also search for this author in PubMed Google Scholar
Contributions
R.T.S. reviewed the literature, drafted and revised the manuscript. D.P., D.C.S., D.C.B., K.I.K., and R.N.F. provided critical input, discussion, and revision of the manuscript. All authors approved the final submitted version of the manuscript.
Corresponding author
Correspondence to Karen I. Kroeker .
Ethics declarations
Competing interests.
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/ .
Reprints and permissions
About this article
Cite this article.
Sutton, R.T., Pincock, D., Baumgart, D.C. et al. An overview of clinical decision support systems: benefits, risks, and strategies for success. npj Digit. Med. 3 , 17 (2020). https://doi.org/10.1038/s41746-020-0221-y
Download citation
Received : 21 February 2019
Accepted : 19 December 2019
Published : 06 February 2020
DOI : https://doi.org/10.1038/s41746-020-0221-y
Share this article
Anyone you share the following link with will be able to read this content:
Sorry, a shareable link is not currently available for this article.
Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by
The association between population health management tools and clinician burnout in the united states va primary care patient-centered medical home.
- Lucinda Leung
- Susan E. Stockdale
BMC Primary Care (2024)
Health professionals’ acceptance of mobile-based clinical guideline application in a resource-limited setting: using a modified UTAUT model
- Addisalem Workie Demsash
- Mulugeta Hayelom Kalayou
- Agmasie Damtew Walle
BMC Medical Education (2024)
Should AI models be explainable to clinicians?
- Gwénolé Abgrall
- Andre L. Holder
- Xavier Monnet
Critical Care (2024)
Clinicians’ roles and necessary levels of understanding in the use of artificial intelligence: A qualitative interview study with German medical students
- S. Tinnemeyer
BMC Medical Ethics (2024)
Title and abstract screening for literature reviews using large language models: an exploratory study in the biomedical domain
- Fabio Dennstädt
- Johannes Zink
- Nikola Cihoric
Systematic Reviews (2024)
Quick links
- Explore articles by subject
- Guide to authors
- Editorial policies
Sign up for the Nature Briefing: Translational Research newsletter — top stories in biotechnology, drug discovery and pharma.
The Role and Importance of Decision Support Systems Essay (Critical Writing)
Short review of the company, using the system to evaluate the proposed level production plan, using the system to assist in annual planning and budgeting, factors for success.
Over the recent years, decision support systems (DSS) have appeared as a new tool for supporting and improving administrative decision-making. A DSS is an interactive computer-based system that offers easy access to choice models and assignments to sustain semi-structured and uncontrolled decision-making tasks. The gradually growing interest in DSS is advanced by supervisors familiar with the accessibility and potentials of this technology.
A DSS creator is a “package” of associated hard and soft that can be used to expand a definite DSS. It naturally holds capabilities such as information and data administration, graphic demonstration, economic and statistical study routines, and optimization analysis-all capabilities that have been obtainable independently for some time, but only lately as an incorporated easy-to-use set of tools. While large company have reclined more upon DSS for successful decision taking, small businesses have practically disregarded this new technology.
The case study offers the investigation of DSS use in some small business company, engaged in the production of plastic toys.
The toy manufacturing is a highly competitive commerce. The sales volumes of the corporation consisted $3 million in 1982, and were planned to be increased up to $3.6 million in 1983. Over 80 percent of annual financial turnover was raised during the August-November time. The company’s manufacturing plan had been seasonal.
In 1982, the company’s production manager became extremely bothered by the issues that arose not only from manufacture planning but also from ultimately falling incomes. Seasonal extension and reduction of the staff resulted in employment troubles as well as high training and quality control expenditure. Equipment, inactive for several months, was unexpectedly subjected to intense use. Speeded up manufacturing plans resulted in regular system modifications on technical equipment and perplexity in planning runs, which reasoned inefficiencies in assemblage and wrapping.
For these grounds, the production manager had recommended the president to assume a strategy of level monthly manufacturing in 1983, indicating that estimations of trade volumes had been steadfast in the earlier periods, and that acquisition terms would not be influenced by the rescheduling of purchases.
The president became aware that an obvious development of manufacture efficiency could result from production levels, but he was hesitant on the issue what the impact on other stages of the industry might be. Predominantly, he was anxious about the effect that productivity level might have on the firm’s prerequisites for finances in 1983. To concentrate on this issue, the production manager offered that ABC needs to develop a computer based DSS to study the financial suggestions of the level production plan.
To correspond acknowledged needs it was determined to elaborate a computer-based DSS that could be implemented into the assistance in yearly planning, to estimate the financial insinuations of the proposed manufacturing level plan, and to assist in cash financial planning.
As it was estimated, net profit ($174,000) gained under the manufacturing level plan is considerably higher than that acquired implementing of the seasonal production plan ($108,000). The monthly documented balance, consequential from the existing manufacturing plan shows that the only noticeable regular financing obligation throughout the year will be a swelling in receivables for the duration of the collection wrap after the peak sales months. The largest expected bank borrowing of $657,000 happens in November, well within the $850,000 credit limit.
The firm’s economic position in July becomes even more dangerous since its existing benefits are contained predominately of inventories, while by September, the increase of accounts receivable is extensive. The risk that takes into account incomes will be uncollectible is much less than the risk that supplies may not be salable, mainly in the toy industry where costs and trends vary sharply in the off-season. The DSS system allows administration to weigh the augmented profit available under a manufacturing level plan against the risk of augmented inventory investment and the complexity of attaining sufficient financing.
The assessment of this kind of trade-off in creation of a production plan resolution is significant when one believes the fact that for a small-scale industry, liquidity and survival are synonymous. Because of predictable complexity in finding sufficient financing, the president ultimately settled on against the level production plan. Nevertheless, the two manufacturing plans appraised above characterization only two extremes of a range of probable substitute manufacturing plans. The administration of ABC Co. realized that the system can be used to decide the most attractive manufacturing plan under the restriction of predictable economic circumstances.
Monthly sales estimates and manufacturing plans are the two main contributions to the process of raising a chain of standard statements. In their annual development procedure, ABC’s administration can use the DSS to test the economic impact of different manufacturing strategies grounded upon an agreed sales estimate. These systems may be created by different groupings of level and seasonal manufacture.
For each manufacturing policy, the model is run by using the “what-if’ characteristic to create a set of monthly pro forma reports. Administration can inspect and analyze the economic influence of these strategies, mainly with value of the necessity for exterior financing. This procedure is duplicated until management is pleased with the projected values on the reports.
Aspects that seem to have donated to the achievement of this scheme are:
- the necessity for a system to sustain top managing decision-making was well recognized;
- top management must support the system from its foundation;
- the production manager needs to serve as the catalyst;
- most important consumers (the vice president of economics and the production manager) vigorously contributed in the actual achievement of the system;
- use of a DSS creator such as IFPS to develop the system.
It should be underlined that a DSS needs to be developed over time in order to better correspond to the requirements of the decision makers and the enterprise. As such, the system has to be updated or modified as the requirements of the users become better recognized or the executive surroundings changes significantly.
Alter, S. L. (1980). Decision support systems: current practice and continuing challenges. Reading, Mass., Addison-Wesley Pub.
Druzdzel, M. J. and R. R. Flynn (1999). Decision Support Systems. Encyclopedia of Library and Information Science. A. Kent, Marcel Dekker, Inc.
Finlay, P. N. (1994). Introducing decision support systems. Oxford, UK Cambridge, Mass., NCC Blackwell; Blackwell Publishers.
Kuang-Chian, C. (1989). Developing Decision Support Systems for Small Business Management: A Case Study. Journal of Small Business Management, 27 (3), 11
Pollock, C., & Kanachowski, A. (1993). Application of Theories of Decision Making to Group Decision Support Systems (GDSS). International Journal of Human-Computer Interaction, 5 (1), 71-94.
- Customer Needs Assessment for Signature Hotels, Toronto
- The Benefits and Problems of Management Empowerment
- DXplain Project Implementation and Evaluation
- Decision-Making System in Digital Firms Management
- The Business Pressure-Response-Support Model
- Common Themes in Total Quality Management
- Comparison of Business Management in India and America
- Effective Managerial Selections Definition
- The Gulf States Metal Inc.: Managerial Problems
- Health Maintenance Organization: Polarities Management
- Chicago (A-D)
- Chicago (N-B)
IvyPanda. (2021, September 20). The Role and Importance of Decision Support Systems. https://ivypanda.com/essays/the-role-and-importance-of-decision-support-systems/
"The Role and Importance of Decision Support Systems." IvyPanda , 20 Sept. 2021, ivypanda.com/essays/the-role-and-importance-of-decision-support-systems/.
IvyPanda . (2021) 'The Role and Importance of Decision Support Systems'. 20 September.
IvyPanda . 2021. "The Role and Importance of Decision Support Systems." September 20, 2021. https://ivypanda.com/essays/the-role-and-importance-of-decision-support-systems/.
1. IvyPanda . "The Role and Importance of Decision Support Systems." September 20, 2021. https://ivypanda.com/essays/the-role-and-importance-of-decision-support-systems/.
Bibliography
IvyPanda . "The Role and Importance of Decision Support Systems." September 20, 2021. https://ivypanda.com/essays/the-role-and-importance-of-decision-support-systems/.
- To find inspiration for your paper and overcome writer’s block
- As a source of information (ensure proper referencing)
- As a template for you assignment
IvyPanda uses cookies and similar technologies to enhance your experience, enabling functionalities such as:
- Basic site functions
- Ensuring secure, safe transactions
- Secure account login
- Remembering account, browser, and regional preferences
- Remembering privacy and security settings
- Analyzing site traffic and usage
- Personalized search, content, and recommendations
- Displaying relevant, targeted ads on and off IvyPanda
Please refer to IvyPanda's Cookies Policy and Privacy Policy for detailed information.
Certain technologies we use are essential for critical functions such as security and site integrity, account authentication, security and privacy preferences, internal site usage and maintenance data, and ensuring the site operates correctly for browsing and transactions.
Cookies and similar technologies are used to enhance your experience by:
- Remembering general and regional preferences
- Personalizing content, search, recommendations, and offers
Some functions, such as personalized recommendations, account preferences, or localization, may not work correctly without these technologies. For more details, please refer to IvyPanda's Cookies Policy .
To enable personalized advertising (such as interest-based ads), we may share your data with our marketing and advertising partners using cookies and other technologies. These partners may have their own information collected about you. Turning off the personalized advertising setting won't stop you from seeing IvyPanda ads, but it may make the ads you see less relevant or more repetitive.
Personalized advertising may be considered a "sale" or "sharing" of the information under California and other state privacy laws, and you may have the right to opt out. Turning off personalized advertising allows you to exercise your right to opt out. Learn more in IvyPanda's Cookies Policy and Privacy Policy .

IMAGES
VIDEO